Between-ward disparities in colorectal cancer incidence and screening in Washington DC
- DOI
- 10.1016/j.jegh.2015.08.001How to use a DOI?
- Keywords
- Colorectal cancer; Incidence; Local data; Risk factors; Screening
- Abstract
This study aims to investigate the incidence and determinants of colorectal cancer (CRC) and its screening in District of Columbia (DC), and identify modifiable risk factors. Data (2000–2009) from the DC Cancer Registry, Behavioral Risk Factor Surveillance System (BRFSS-DC) and Surveillance Epidemiology and End Results (SEER) were used to estimate CRC incidence in eight DC Wards. Risk factors and CRC screening were analyzed using uni-, bi-, and multivariable statistical methods with survey procedures in SAS (version 9.2) including binary, unconditional multivariable logistic regression analysis. Factors measured included stage of diagnosis, age, gender, race/ethnicity, smoking, alcohol, exercise, body weight, health insurance, education, employment, and income. Over the study time, CRC screening increased from 48.4% to 68.6%. Mean age at diagnosis was 67 years. CRC incidence is high in DC. Furthermore, CRC incidence rates in DC below 50 years age were higher than the SEER18 average. Disparities exist between CRC incidence and screening among DC Wards. Identified risk factors for CRC are smoking, obesity, and low physical activity; screening was less prevalent among the uninsured and low socio-economic group. Local variations in CRC occurrence exist and may vary from average national experiences. Identification of local regions which vary from national trends in disease occurrence is important for comprehensive understanding of the disease in the community.
- Copyright
- © 2015 Ministry of Health, Saudi Arabia. Published by Elsevier Ltd.
- Open Access
- This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
In the United States (US), colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second leading cause of cancer associated deaths [1]. It is estimated that in 2015, some 132,700 men and women will be diagnosed with CRC and 49,700 deaths will be attributable to CRC [2]. CRC cost an estimated 14 billion dollars in 2010 ($15.27 billion in 2015 inflation adjusted dollars), accounting for almost 11% of the total cost of cancer care [3]. Predicted national expenditures in 2020, by phases of care, indicate that among all major cancers, CRC will have the highest expenditure in its initial phase of care, despite its decreasing incidence [3].
Recent incidence and mortality rates of CRC among adults older than 50 years of age have declined, but can be improved further. Most CRC cases arise from premalignant adenomatous polyps through an adenoma-adenocarcinoma transformation that takes 7–15 years [4]. CRC can be prevented by early detection and polyp removal [4]. Whereas the 5-year relative survival for CRC between 2002 and 2008 was approximately 60% [2], the rate for localized diseases was higher (90%) [2]. Distant metastasized (Stage IV) tumors had a 5-year survival of only 11% [2].
The American Cancer Society and the US Multi-Society Task Force recommend routine CRC screening for all average risk adults beginning at age 50 years by one of the following options: (1) annual fecal occult blood test (FOBT) or fecal immunochemical test; (2) 5-yearly flexible sigmoidoscopy; (3) 10-yearly colonoscopy; (4) 5-yearly double-contrast barium enema; (5) 5-yearly computed tomography colonography; or (6) sDNA [5].
CRC incidence rates below the recommended screening age are increasing [6]. The rise in CRC incidence among the younger population has been attributed to behavioral risk factors such as consumption of red meat, lack of physical activity, smoking, and alcohol consumption [6]. Several studies have demonstrated a role of lifestyle modification and reduction of modifiable risk factors in primary prevention of CRC. The microsimulation CRC screening analysis model (MISCAN-Colon) estimated the impact of historic changes in risk factors, screening, and treatment on CRC incidence and mortality trends, and established the role of physical inactivity, overweight and obesity, and a diet high in red and processed meat, as major modifiable risk factors for CRC [7].
The decline in CRC incidence and mortality over the past few decades has largely been attributed to increased screening among average risk adults 50 years and older [7]. Access to CRC screening is likely to increase with The Patient Protection and Affordable Care Act of 2010. However, the current screening guidelines do not address CRC in individuals younger than 50 years of age [8], and at this time, evidence to support population-wide screening in this age group is weak [8]. With the rising number of CRC cases in younger adults and a lack of screening guidelines in this age group, it is important to pay greater attention to behavioral risk factors and formulate potential prevention strategies. Thus, primary prevention of CRC through screening as well as lifestyle modification may be an effective strategy for both older and younger adults [9].
1.1. Status of CRC in Washington DC
The overall 5-year rate changes show a decreasing trend in incidence of CRC in the District of Columbia (DC) over time, with an estimated annual percentage change (APC) of −4.0% from 2003 to 2007. However, despite its declining trend, DC carries one of the highest incidence rates of CRC in the US [10]. The incidence rate of CRC in DC in 2009 was 43.8/100,000 population as compared with 42.3/100,000 population in the US, and higher than states such as Arizona, Colorado, Florida, Maryland, Oregon, Utah, and Washington (average of 37.2/100,000 population) [11]. The incidence rate was higher among Blacks as compared with Whites in DC in 2009 (53.9/100,000 population vs. 24.8/100,000 population) [11].
DC is divided into eight distinct geographical units called Wards (numbered 1–8) which elect their representatives as members of the DC City Council. There are four cancer centers, 11 hospitals, and several excellent cancer care services in DC [12]. However, a high prevalence of cancer continues to persist in DC, because many of these services are neither accessible nor affordable for the poor and medically underserved (uninsured/underinsured) DC residents [12]. The divergent trend in CRC incidence and mortality between Blacks and Whites in DC has usually been explained by late stage diagnosis [13], and a complex interplay of clinical, social, biological, and environmental factors [14]. It is therefore important to understand tumor biology, genetics, and sociodemographic and lifestyle risk factors among DC residents to understand these disparities.
1.2. Objectives
Despite the large amount of scientific literature on CRC, population-based studies in DC describing epidemiology, estimate of disease magnitude, its distribution, and local risk factors are few. Much undetermined basic CRC epidemiologic information remains in DC such as estimates of the affected population in each DC Ward, Ward-specific screening estimates, small area variations, and modifiable risk factors impacting incidence and CRC outcome. The overall goal of this study was to study the distribution and determinants of CRC in DC and its Wards, and to identify geographical areas and modifiable lifestyle factors as intervention targets. We report the results following the STROBE guidelines.
2. Methods
Data for this study came from three distinct sources: (1) the Washington DC cancer registry (DCCR) (years 2000–2009); (2) the Behavioral Risk Factor Surveillance System (BRFSS 2002–2008); and (3) the US Census Bureau. Data from Surveillance Epidemiology and End Results (SEER) were used for comparison between DC and the SEER-18 states. DCCR collects cancer data on all cancers diagnosed and/or treated in DC by tracking the incidence and mortality of all types of cancers. The DCCR gathers its data from hospitals, laboratories, and other reporting agencies in DC, as well as its neighboring states, to capture all occurrences of cancer among DC residents.
Age adjustments were based on the US 2000 population. Cumulative and yearly CRC incidences were calculated using all CRC cases reported in the DCCR, with US Census Bureau (2000) DC resident population as the denominator. The US Census Bureau’s Population Estimates Program produces estimates of the population and population changes for the US every year in states, counties, cities, and towns. With every new annual estimate release, the entire time series of estimates is revised for all years back to the last census.
DC Ward-specific CRC screening prevalence (FOBT and sigmoidoscopy/colonoscopy together referred to in this report as colonoscopy), and behavioral and socioeconomic determinants were analyzed from the BRFSS. Because cases of CRC are rare below the age of 20 years, analysis was restricted to populations aged 20 years and above. This HIPAA-compliant study was cleared by The George Washington University Institutional Review Board.
2.1. Statistical analysis
The main outcome variable was: receipt of CRC screening tests (yes/no). Independent variables included age categories (20–49 years, 50–64 years, 65–74 years, and ⩾75 years), gender (men/women), health insurance (yes/no), education (never attended or elementary school, attended school, college), annual income (<$50,000, $50,000 to $74,000, ⩾$75,000), employment (employed, unemployed, others [retired, housewives, students]) and calculated body mass index.
We determined associations between variables using differences between means, odds ratios (ORs), and 95% confidence intervals (CI) (two-tailed tests with significance level set at α = 0.05). Due to the multistage cluster sampling design of the BRFSS survey (to account for design effect and obtain appropriate estimates for standard errors), we conducted our analysis by employing survey procedures in PC-SAS/STAT (V9.2, SAS institute, Cary, NC, USA). We generated GIS maps with appropriate overlays for data display using ArcGIS (V9.2) software. After univariate analysis to check for data distribution, completeness and consistency, we used t tests to compare the mean differences in CRC incidence between the US and SEE-18 states and between men and women in DC. We used Chi-square tests to assess the association between independent and outcome variables. We evaluated prediction of CRC screening practices by independent variables employing binary, unconditional multivariable logistic regression analysis.
3. Results
3.1. Distribution of CRC in DC
The 2000–2009 study data file included all 3534 CRC cases reported to the DCCR. Table 1 describes demographic characteristics of CRC patients in DC. The highest incidence rate (356.9/100,000) was seen among those >75 years of age. The incidence of CRC was highest among Blacks (71.0/100,000 in Blacks compared with 44.8/100,000 in Whites and 58.7/100,000 among other races).
| Factor | Level | n (%) in DC | CRC incidence in DCa | CRC incidence in the U.S.a | Screening prevalence in DC Wards (%) | |
|---|---|---|---|---|---|---|
| FOBT | Colonoscopy | |||||
| Age groups (y) | 20–49 | 380 (10.8) | 13.5 | 10 | – | – |
| 50–64 | 1158 (32.8) | 135.1 | 78.1 | – | – | |
| 65–74 | 780 (22.1) | 217.1 | 182.6 | – | – | |
| ⩾75 | 1213 (34.3) | 356.9 | 302.7 | – | – | |
| Sex | Male | 1662 (47.0) | 61.7 | 58 | – | – |
| Female | 1862 (52.7) | 61.5 | 42.7 | – | – | |
| Race | White | 788 (22.4) | 44.8 | 48.6 | – | – |
| Black | 2432 (69) | 71 | 59.9 | – | – | |
| Others | 304 (8.6) | 58.7 | 40.2 | – | – | |
| DC Wards | Ward 1 | 325 (9.5) | 44.3 | – | 55.2 | 66.3 |
| Ward 2 | 318 (9.3) | 46.2 | – | 63.1 | 70.6 | |
| Ward 3 | 411 (12.1) | 55.7 | – | 66.7 | 74.2 | |
| Ward 4 | 507 (14.9) | 68.4 | – | 54.3 | 68.4 | |
| Ward 5 | 651 (19.1) | 90.0 | – | 50.8 | 60.7 | |
| Ward 6 | 420 (12.3) | 61.7 | – | 58.2 | 68.0 | |
| Ward 7 | 442 (13.0) | 62.7 | – | 47.7 | 63.7 | |
| Ward 8 | 351 (10.3) | 49.5 | – | 40.2 | 52.4 | |
FOBT = fecal occult blood test.
Incidence/100,000 populations per year.
Demographic characteristics, Ward-wise incidence of and screening for colorectal cancer (CRC) in District of Columbia (DC) (cumulative: year 2000–2009).
The incidence of CRC in DC decreased for all races and genders from 2000 to 2009 (Fig. 1). Although the incidence of CRC substantially increased in 2002, it showed a generally decreasing trend thereafter (from 2003 to 2009). We attribute this “spike” to a small number of cases resulting in unstable rates at times of demographic changes (between 2000 and 2005, population of Blacks in DC decreased by 3.24% and those of Whites, Latinos, and other races increased, respectively, by 1.85%, 1.055%, and 2.16%; data available from the US Census Bureau). The APC in CRC incidence varied from year to year, with the largest change (−3.5%: data not shown) occurring between 2003 and 2004. The average age adjusted cumulative incidence of CRC in DC (2002–2009) was significantly higher than the national average (p = 0.04). However, on a year-to-year comparison, this difference disappeared from 2006 onwards (Fig. 1). Although a sharp rise in cumulative incidence of CRC was noted among Whites in 2003, this was not statistically significant (p = 0.08). The CRC incidence in Blacks was significantly higher compared with Whites (p = 0.03) in DC. The median age of CRC diagnosis in DC was 65 years in men and 70 years in women. There was no significant difference in CRC incidence between men and women in DC (p = 0.89) (Fig. 1).
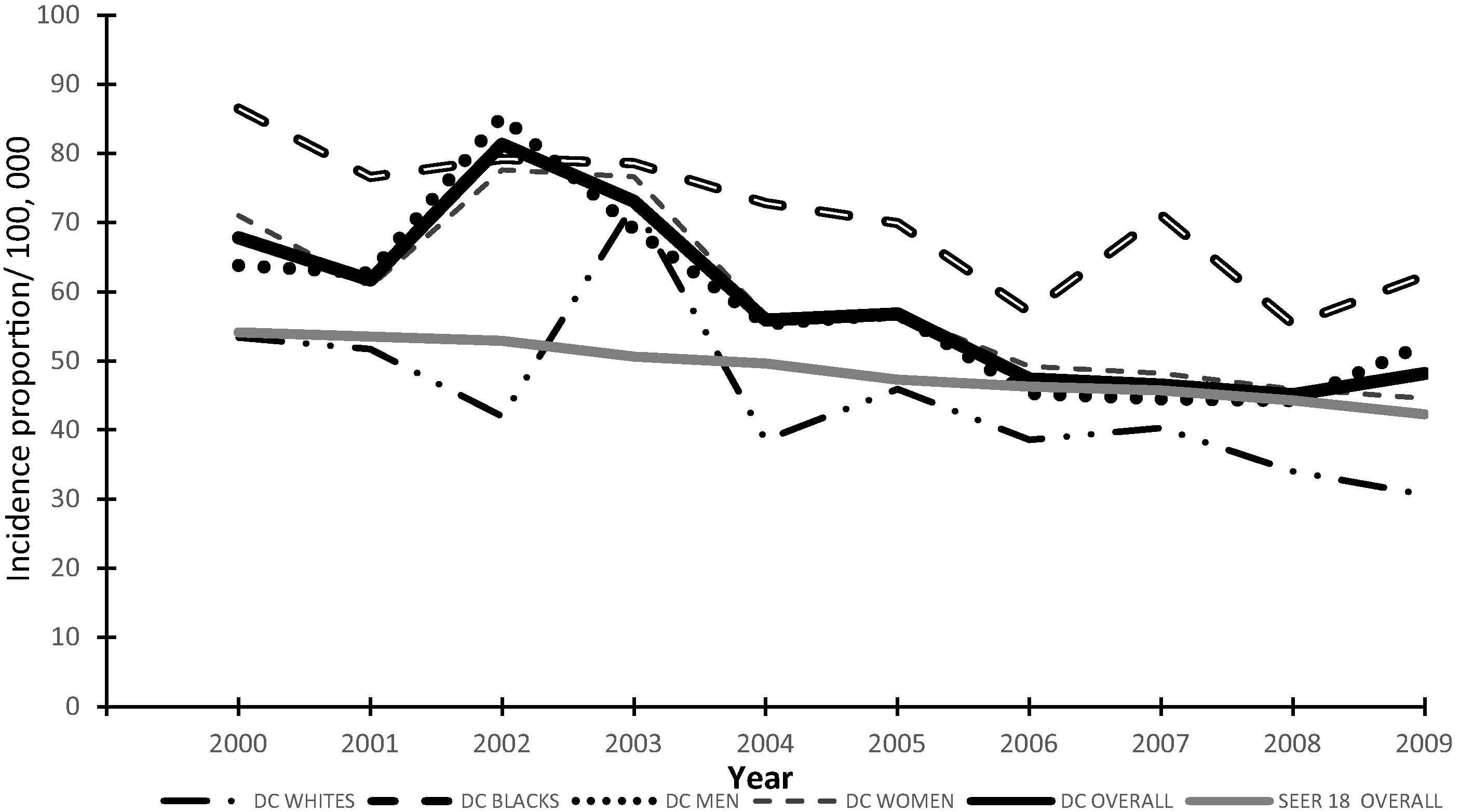
Race and gender distribution of incident colorectal cancer (CRC) in Washington, District of Columbia (DC) and overall comparison with Surveillance Epidemiology and End Results (SEER) 18, years 2000–2009.
3.2. Distribution of CRC in Wards
Overall, the cumulative and yearly (2000–2009) incidence of CRC was highest in Ward 5 (Table 1). The CRC incidence increased in Ward 5 and Ward 8 between 2007 and 2009. Approximately 29% of tumors were in Stage IV; 21.2% of these Stage IV tumors were reported from Ward 5, and 15.4% from Ward 8. Ward 3 had the lowest prevalence of Stage IV tumors (7.4%), whereas Ward 8 had the lowest prevalence of localized tumors (8.5%).
3.3. Distribution of CRC screening in DC
CRC screening analysis included 13,139 BRFSS respondents who answered questions on FOBT (n = 6578) or colonoscopy (n = 6561). Altogether, 57.7% of the respondents reported time-interval appropriate CRC screening by at least one method. Of those who had FOBT, 48.5% reported having been tested within 1 year and 94% of colonoscopy respondents underwent testing in the past 5 years. DC showed an increasing trend in colonoscopy, (60.2% in 2002 to 71.2% in 2008), and a slightly declining trend in FOBT (49.5% in 2002 to 46.5% in 2008). No CRC screening was reported in the 20–49 years age group. The odds of being screened for CRC with FOBT or colonoscopy were highest in the 65–74 years age group compared with 50–64 years age group (OR 1.4, 95% CI: 1.2–1.7; and OR 2.3, CI: 1.9–2.7), and was lower among the uninsured compared with the insured (FOBT: OR 0.4, CI: 0.3–0.5; colonoscopy OR 0.3 CI: 0.2–0.4) (Table 2). Despite 92% health insurance coverage in DC, only 51.8% of DC residents covered under insurance underwent FOBT and 62.9% underwent colonoscopy. Screening was significantly less likely among elementary and high school graduates as compared with college graduates (FOBT: OR 0.3, CI: 0.2–0.7 and OR 0.6, CI: 0.5–0.9; colonoscopy: OR 0.4, CI: 0.3–0.5 and OR 0.6, CI: 0.5–0.7) and in the low income group compared with the high income group (OR 0.6, CI: 0.4–0.7 and OR 0.5, CI: 0.4–0.6).
| Characteristic | Level | Crude | Adjusted model | ||
|---|---|---|---|---|---|
| FOBT | Sigmoid/colonoscopy | FOBT | Sigmoid/colonoscopy | ||
| Age (y) | 50–64 | 1 | 1 | 1 | 1 |
| 65–74 | 1.4 (1.2, 1.7)* | 2.3 (1.9, 2.7)* | 1.6 (1.4, 1.9)* | 2.6 (2.2, 3.0)* | |
| ⩾75 | 1.1 (0.9, 1.4) | 1.6 (1.4, 1.9)* | 1.4 (1.1, 1.6)* | 1.7 (1.4, 2.1)* | |
| Gender | Men | 1 | 1 | 1 | |
| Women | 1.0 (0.8, 1.2) | 1.0 (0.8, 1.2) | 1.1 (0.9, 1.2) | 1.2 (1.0, 1.3) | |
| Health insurance | Yes | 1 | 1 | 1 | 1 |
| No | 0.4 (0.3, 0.5)* | 0.3 (0.2, 0.4)* | 0.6 (0.4, 0.8)* | 0.5 (0.4, 0.6) * | |
| Education | Elementary | 0.3 (0.2, 0.7)* | 0.4 (0.3, 0.5) * | 0.4 (0.3, 0.6)* | 0.4 (0.3, 0.6)* |
| School | 0.6 (0.5, 0.9)* | 0.6 (0.5, 0.7)* | 0.7 (0.6, 0.9)* | 0.6 (0.5, 0.7)* | |
| College | 1 | 1 | 1 | 1 | |
| Income | Low | 0.6 (0.4, 0.7)* | 0.5 (0.4, 0.6)* | 0.6 (0.5, 0.7)* | 0.5 (0.4, 0.6)* |
| Moderate | 0.7 (0.5, 0.8)* | 0.7 (0.5, 0.8)* | 0.7 (0.6, 0.8)* | 0.7 (0.5, 0.8)* | |
| High | 1 | 1 | 1 | 1 | |
| Employment | Employed | 1 | 1 | 1 | 1 |
| Unemployed | 0.6 (0.4, 0.8)* | 0.5 (0.4, 0.7)* | 0.8 (0.6, 1.1) | 0.7 (0.5, 0.9)* | |
| Others | 1.0 (0.9, 1.1) | 1.3 (1.2, 1.5)* | 0.9 (0.8, 1.1) | 1.3 (1.1, 1.5)* | |
Statistically significant difference from reference group.
Odds ratios [95% confidence interval (CI)] from binary, unconditional crude, and multivariable (adjusted) logistic regression analysis for fecal occult blood test (FOBT) and colonoscopy in District of Columbia (DC).
3.4. Distribution of CRC screening in Wards
Colonoscopy prevalence in eight DC Wards over years 2002–2008 showed an increasing trend of screening prevalence in Ward 1 (58–78.6%), Ward 2 (62.6–76.8%), and Ward 3 (65.9–83.2%); other Wards showed year-wise fluctuations. Overall, Ward 3 had the highest (74%) and Ward 8 had the lowest (51.8%) CRC screening prevalence. Ward 5, with the highest incidence of CRC, had an overall screening prevalence of 60.6%. Fig. 2 summarizes Ward-wise cumulative CRC incidence and screening prevalence in DC (2000–2009).
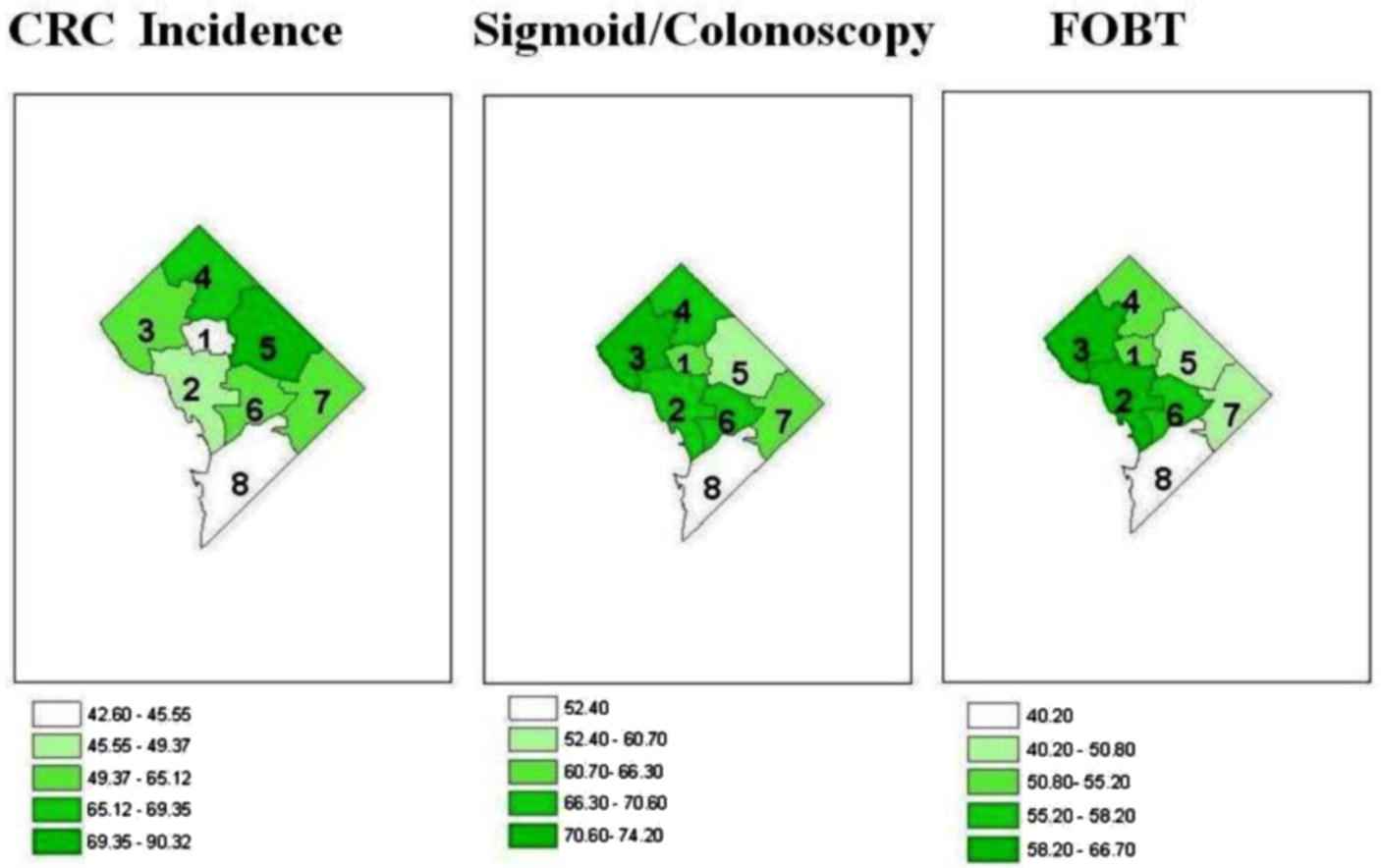
District of Columbia (DC) Ward-wise cumulative distribution (2000–2009) of colorectal cancer (CRC) incidence and CRC screening prevalence.
3.5. CRC and CRC screening associated risk factors
Logistic regression analysis (Table 2) suggested that age group, education, health insurance, employment, and income were independently associated with FOBT and colonoscopy screening in DC. Upon comparing ORs between crude and multivariable adjusted models, most ORs increased in the adjusted models (range of increase between 7% and 67% for colonoscopy; and between 10% and 53% for FOBT). The values of the CIs of the effect estimates and their precision did not change appreciably.
Some important risk factors associated with CRC were analyzed according to their prevalence in eight DC Wards. The population and sociodemographic characteristics were different across all Wards (p < 0.0001). Ward 3 had a high proportion of the population older than 65 years (13.8%), who were mostly Whites (83.6%), with the highest percentage of college graduates (79.1%), and a median annual income of $71,875 [15]. The proportion of current smokers in Ward 3 was less as compared with others. In contrast, Ward 7 (97% Blacks, 43.9% men and 56.1% women, 14% >65 years of age, median annual income of $30,533) [15] and Ward 8 (92.4% Blacks, 44.8% men and 55.2% women, 6.4% >65 years of age, median annual income of $25,017) [15] had the lowest proportion of college graduates (12.6% and 8%), low physical activity levels (90% in Ward 3 vs. 65% in Ward 7, and 67% in Ward 8) and high prevalence of obesity (72% in Ward 7 and 71% in Ward 8).
4. Discussion
CRC is the fourth leading cause of cancer in DC, ranking after prostate, breast, and lung cancers [12]. Our study shows that incidence of CRC, although showing an overall decline had increased among Whites in DC in 2002–2003. This could be interpreted in context of federal changes in insurance coverage for CRC screening. In July 2001, the federal government added colonoscopy screening as a covered service for “average risk” Medicare beneficiaries, thereby equalizing coverage of tests across all insurance groups [16]. It is also well documented that in general, more Whites undergo CRC screening than Blacks [10]. This higher “background” colonoscopy screening among Whites may also explain the Black–White disparity in CRC incidence in DC during that period. Although the rates of CRC in the US have been declining, Blacks in DC have not experienced such a decline. CRC incidence in DC was also higher than national figures for those between 20 and 49 years of age. A recent large population based study conducted to describe CRC burden in adults below 50 years of age concluded that almost 8% of CRC cases occurred in persons of this age group [17]. Results of a cancer surveillance project in Canada were also similar [18]. In the US, Blacks present with CRC at a younger age than Whites [19]. Recently, The American College of Gastroenterology recommended a lower CRC screening age in Blacks from 50 to 45 years because of the lower mean age of CRC incidence among Blacks [20]. Our observation of higher CRC incidence in the younger age group in DC could be due to the proportionately larger Black population in DC (61.7%). The high incidence of CRC among the young in DC demonstrates a need for further exploration of CRC risk factors and screening practices in this group.
Results of this study have consistently shown the highest yearly prevalence of CRC in Ward 5 over the examined 8-year period (86.7% Blacks, 46.7% men and 53.3% women, 17.8% >65 years of age, median income of $34,433 annually) [15]. In a recent study, Oliver et al. [15] discussed a strong association of income with colonoscopy procedures, demonstrating that CRC screening procedures are low among lower income African American males. Competing demands within a limited family income and test-related out-of-pocket costs reduce patients’ interests in screening [21]. We are thus able to hypothesize that a difference in preventive care-seeking behavior may have led to the high incidence of CRC in Ward 5. Further studies should be able to test this hypothesis.
Data from this study identified Ward-specific variability for CRC stage of diagnosis. The differences in stage of tumor diagnosis may be attributable to delays in diagnosis caused by late patient presentation, lack of insurance, and low income, which delay seeking medical care [22]. Previous studies have shown that Blacks are more likely to present with late stage tumors than Whites [19]. Our analysis showed that 76% of Stage IV tumors occurred among Blacks, and the majority were diagnosed in Ward 5 and Ward 8. This emphasizes the need to improve awareness about CRC in these Wards and for more focused efforts to increase screening and early detection in Ward 5 and Ward 8.
A high prevalence of Stage IV tumors in Ward 8 could also be due to limited screening resources in this Ward, hindering early diagnosis. Evidence suggests that physicians are hesitant to order even a FOBT, if adequate colonoscopy resources are not available to follow-up the positive FOBT results [23,24]. Our study findings are consistent with these reports, as we find a low incidence of CRC in Ward 8, but a high proportion of Stage IV tumors. A high-sensitivity FOBT that can detect a majority of prevalent CRC in an asymptomatic population can be an acceptable option for CRC screening in average-risk adults aged 50 years or above in these Wards if they have colonoscopy resources to follow up positive FOBT tests.
Current efforts in CRC prevention in the US focus primarily on screening and removal of any precancerous polyps in individuals at or above 50 years of age. Because persons below 50 years of age are less likely to be screened for CRC, attention should be given to preventing disease in younger adults by addressing modifiable CRC risk factors. While increasing physical activity and maintaining a healthy weight can decrease the risk for CRC by almost 50% [25], smokers have two- to threefold elevated risk of colorectal adenoma, which are precursors of CRC [26]. We identified DC Wards with a greater prevalence of CRC risk factors. The highest prevalence of smoking, obesity, and low physical activity was in Wards 5–8. The confluence of multiple CRC risk factors could be the main drivers of high CRC incidence in Wards 5, 7, and 8.
CRC screenings using flexible sigmoidoscopy and colonoscopy have been consistently associated with lower CRC incidence and mortality [27,28]. Our study showed that health insurance, education, employment, and income were independently associated with CRC screening. Similar findings were reported from a study conducted in two Danish counties with unemployment, low income, and a low level of education seen to be significantly associated with participation in CRC screening [29]. Other studies examining determinants of CRC screening behavior also documented education, income, and health insurance to be important CRC screening predictors [30].
Our study is limited by the fact that comparisons between data from the BRFSS and DCCR may be ecological in nature, because the screening and cancer incidence were not measured in the same individual or in a cohort study. However, the goal of this study was to assess screening practices in DC and not to assess a causal association between screening and incidence of CRC. The goals of the study were well addressed. Furthermore, the BRFSS, being a land line telephonic survey, may not truly represent the entire DC population or individual ward populations. Also, data for receipt of CRC screening were all self-reported and not confirmed by reviewing medical records. Survey responses were relatively low for screening and the BRFSS questionnaire did not distinguish between diagnostic and screening procedures. However, this is the first population-based study in DC evaluating Ward-specific epidemiology of CRC which provides important information about CRC status in DC. National level data about disease incidence are often available and discussed. Through our study, we were able to demonstrate that local variations exist and national policies may not always be applicable to local situations. It is also possible that disease experiences in specific communities may vary from national experiences and may even contradict national trends. Although the overall CRC incidence rate has been decreasing in the US and DC, CRC incidence rates increased during the period 2007 to 2009 in Wards 5, 7, and 8 in DC. More recent data are now under evaluation. Such local variations may also exist in other metropolitan statistical areas in the US. Despite improved insurance coverage and access to care in DC, CRC screening services are underutilized, leading to high incidence and late stage CRC, with disparities between CRC screening efforts and CRC incidence in DC. Our study was able to identify areas in DC for more targeted screening and educational campaigns and also argue for developing evidence-based policy making and implementation for CRC screening in DC.
5. Conclusion
Overall incidence of CRC has declined and whole CRC screening in DC has increased over time. The screening progress is slow despite expansion of insurance coverage. Wards 4, 5, 7, and 8 and the Black female population in DC need to be targeted for improved screening practices and healthier lifestyle interventions. Ongoing challenges include lack of public awareness about CRC, its risk factors, and screening practices. A prudent approach to disparity eradication should consider causes for local variations in disease occurrences which are different from the national average.
Conflicts of interest
None of the authors for this article have any professional or financial conflicts of interest.
Acknowledgments
This study was supported in part from funding through The George Washington University, Department of Epidemiology and Biostatistics Practicum Research Fellowship Award, 2011. The authors gratefully acknowledge the support from Dr. Amari Pearson-Fields, Alicia Vargas and Kathleen Rogers of the District of Columbia Department of Health, USA with access to data and general guidance.
References
Cite this article
TY - JOUR AU - Sharmila Chatterjee AU - Amit Chattopadhyay AU - Paul H. Levine PY - 2015 DA - 2015/09/04 TI - Between-ward disparities in colorectal cancer incidence and screening in Washington DC JO - Journal of Epidemiology and Global Health SP - S1 EP - S9 VL - 5 IS - Supplement 1 SN - 2210-6014 UR - https://doi.org/10.1016/j.jegh.2015.08.001 DO - 10.1016/j.jegh.2015.08.001 ID - Chatterjee2015 ER -
