Dexamethasone as a Treatment of COVID-19
 , Mahmoud Kandeel1, 3, *
, Mahmoud Kandeel1, 3, *- DOI
- 10.2991/dsahmj.k.201125.001How to use a DOI?
- Keywords
- COVID-19; SARS-CoV-2; cytokine storm; dexamethasone
- Copyright
- © 2020 Dr. Sulaiman Al Habib Medical Group. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
Dexamethasone is a potent synthetic corticosteroid that possesses anti-inflammatory and immunosuppression effects, which are commonly used to prevent chemotherapy-induced nausea and vomiting, and to treat acute exacerbation of multiple sclerosis, cerebral edema, shock, severe allergies, and multiple myeloma [1]. Unlike some synthetic corticosteroids such as prednisolone, methylprednisolone, and hydrocortisone, dexamethasone is a selective stimulator of glucocorticoid receptors and does not exert any mineralocorticoid activity [2].
Dexamethasone acts as a stimulator of glucocorticoid receptors signaling pathway, which reduces the production of cytokines and decreases neutrophil tissue invasion and damage [3]. In addition, dexamethasone inhibits the phospholipase A2 enzyme that plays a role in the biosynthesis of leukotrienes, prostaglandins, thromboxane A2, and prostacyclin [4]. The bioavailability and volume of distribution of dexamethasone dose is high via the oral, intramuscular, and intravenous routes [5,6]. It is mainly metabolized in the liver by the CYP3A4 enzyme to produce 6alpha- and 6beta-hydroxydexamethasone [7]. The plasma half-life of dexamethasone is about 3 h; however, its biological half-life is much longer (up to 54 h) [8].
Previous studies have shown that glucocorticoid stimulators could be effective and safe drugs in treating severe pulmonary diseases by reducing inflammatory status through inhibiting the gene expression of several cytokines; thus, highly potent glucocorticosteroids may be able to deter the cytokine storm syndrome and minimize the chance of multiorgan failure [9,10]. However, the glucocorticosteroids’ immunosuppressive effect during severe pulmonary diseases caused by bacterial or viral infection could pose as a barrier to the pathogen elimination mechanism by inducing lymphocyte apoptosis and inhibition of T and B cells [11].
Dexamethasone and other corticosteroids were used to treat severe respiratory diseases caused by the influenza virus, Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), and Middle East respiratory syndrome coronavirus. However, all corticosteroids did not lead to any reduction in mortality rate among infected individuals with severe conditions, and could be related to increasing the duration of viral infections and longer hospital stays based on data from observational studies [12,13].
During the disease outbreak in 2020 related to the viral infection caused by a new strain of betacoronavirus called SARS-CoV-2, corticosteroids including dexamethasone were used to treat severe cases of Coronavirus Disease 19 (COVID-19) (Figure 1); however, the routine use of glucocorticosteroids was extremely controversial because of the conflicting results of various studies conducted on the use of corticosteroids for treatment of COVID-19. Two observational studies concluded that corticosteroids could increase the mortality rate among patients with severe COVID-19. By contrast, one cohort study conducted in the UK, in which 6 mg of oral or intravenous dexamethasone daily for 10 days was administered to 2104 COVID-19 patients, showed a reduction in the mortality of more than 30% of ventilated patients, compared with patients who did not receive any type of corticosteroids [14]. A recent study performed in São Paulo, Brazil, included 299 patients diagnosed with COVID-19 and having moderate to severe Acute Respiratory Distress Syndrome (ARDS). Out of 299 COVID-19 patients, 151 received 10–20 mg intravenous dexamethasone until intensive care unit discharge. Even though there was no significant difference in all-cause mortality between patients who received dexamethasone and patients who received standard care only, there was a significant increase in the number of mechanical ventilation-free days over 28 days in the dexamethasone group [15]. Currently, there are two Phase 3 clinical trials for evaluation of dexamethasone in treating COVID-19. The first, which is conducted by Hospital Ana Nery in Salvador, Bahia, Brazil, is a large randomized clinical trial involving patients with moderate and severe ARDS caused by SARS-CoV-2. The second one, performed by Marie Lannelongue Hospital Le Plessis-Robinson, (France), evaluates the efficacy of dexamethasone–hydroxychloroquine combination in treating ARDS caused by COVID-19 [16].
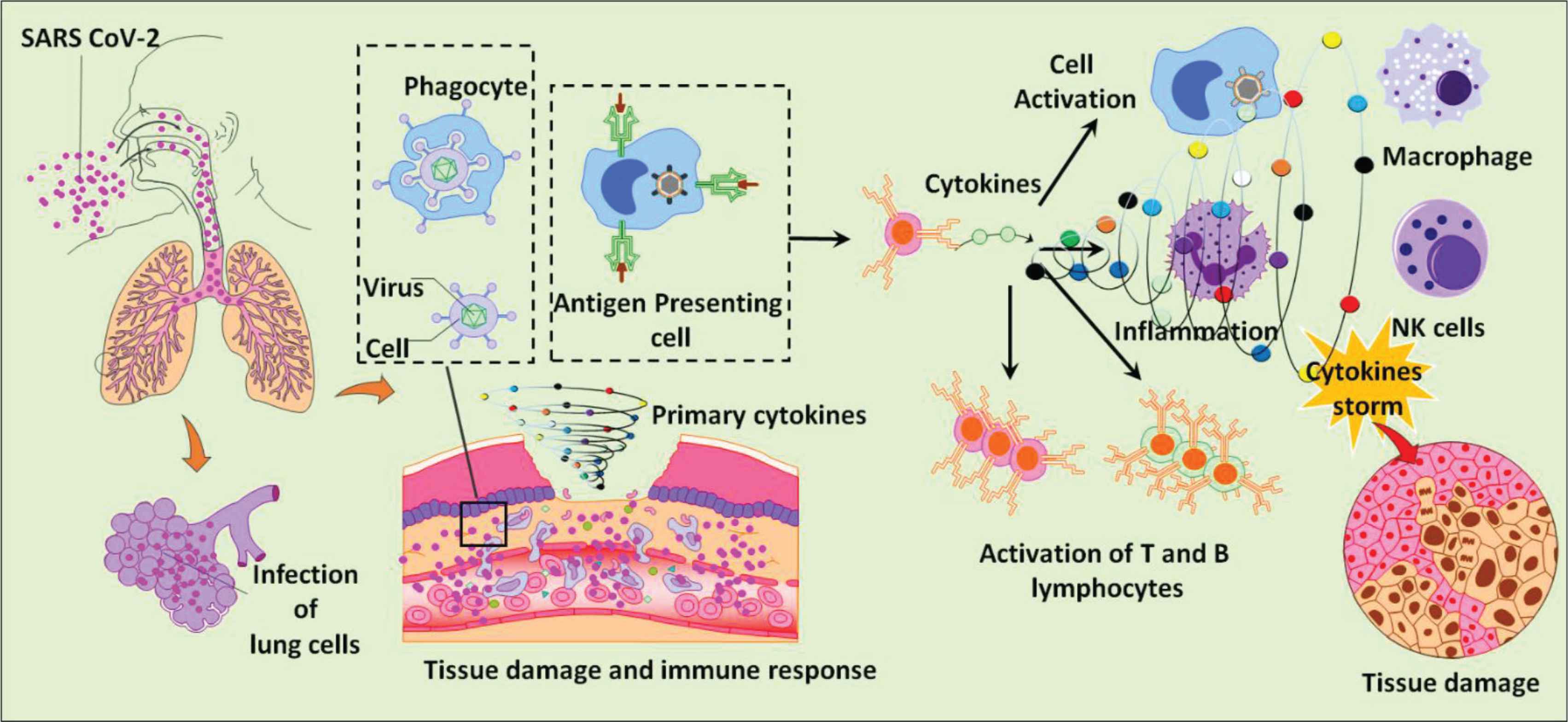
The cellular reaction to lung infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and its associated cytokine storm.
The side effects of dexamethasone during the treatment of coronaviruses were the drawback of its use and resulted in poor prognosis in specific cases. Hyperglycemia was one of the main adverse effects of dexamethasone among diabetic and prediabetic patients, and these patients probably need to take insulin at high doses in order to overcome the hyperglycemic state and to avoid diabetic complications. Another main side effect of dexamethasone was hypertension, which could increase the risk of a new Cardiovascular Event (CVE) among individuals who have risk factors or previous CVE [17].
Overall, dexamethasone so far appears to be a safe and effective treatment option for moderate or severe respiratory disease caused by COVID-19.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
AA and MK contributed in conceptualization. AA contributed in writing – original draft preparation. MK contributed in writing – review and editing. AA and MK contributed in resources. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENT
MK received financial support from
REFERENCES
Cite this article
TY - JOUR AU - Abdullah Alkattan AU - Mahmoud Kandeel PY - 2020 DA - 2020/12/03 TI - Dexamethasone as a Treatment of COVID-19 JO - Dr. Sulaiman Al Habib Medical Journal SP - 7 EP - 9 VL - 3 IS - 1 SN - 2590-3349 UR - https://doi.org/10.2991/dsahmj.k.201125.001 DO - 10.2991/dsahmj.k.201125.001 ID - Alkattan2020 ER -
