Convalescent Plasma Therapy in First Four Critically Ill COVID-19 Patients in Kerala, India

- DOI
- 10.2991/dsahmj.k.200908.001How to use a DOI?
- Keywords
- COVID-19; plasma therapy; Kerala; India
- Abstract
We describe the first four coronavirus disease 2019 (COVID-19) cases in Kerala—the Indian state that reported the first COVID-19 case on January 30, 2020—who developed acute respiratory distress syndrome despite lopinavir/ritonavir and methyl prednisolone treatment. All four patients received convalescent plasma therapy. All four patients showed clinical improvement in 24–48 h and were discharged from hospital after negative severe acute respiratory syndrome coronavirus 2 reverse transcription-polymerase chain reaction test results.
- Copyright
- © 2020 Dr. Sulaiman Al Habib Medical Group. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
The first case of infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in India was reported from Kerala on January 30, 2020 in a student returnee from China. In India, Kerala (population, 35 million)—a state with storied high performance in public health and social development but with low gross domestic product—was the first to establish comprehensive SARS-CoV-2 epidemic control [1]. With the highest level of political stewardship, population compliance, and civic society partnerships, Kerala’s SARS-CoV-2 epidemic control efforts evolved to one of the most successful Coronavirus Disease 2019 (COVID-19) mitigation strategies in India, demonstrated by sustained stemming of extensive local transmission from the state’s returning guest worker population from overseas and the rest of India. As of June 24, 2020, in Kerala, COVID-19 was mainly detected among its returning population, cases remained below 100, and local transmissions were contained.
Given the lack of specific treatments for COVID-19, on March 24, 2020, the United States Food and Drug Administration approved the use convalescent plasma in patients with serious or life-threatening COVID-19. On April 20, India’s Central Drugs Standard Control Organization followed suit [2–8]. However, these approvals are based on a few studies from countries with early outbreak of COVID-19, and their utility in settings such as India remains to be assessed. Therefore, individual state authorities in India, such as Kerala State Medical Board [9] and participating treatment sites issued ethical guidance for compassionate use of convalescent plasma including at the Manjeri Medical College (MMC). In Kerala, MMC is among the eight advanced care institutions designated for COVID-19, one of the centers to initiate plasma therapy early in the course of the COVID-19 outbreak. We report on the first four cases of severe COVID-19 patients with Acute Respiratory Distress Syndrome (ARDS) in Kerala. Consent for the abstraction and use of clinical data from COVID-19 patients is obtained routinely and retained at the MMC.
2. FINDINGS
All four patients had returned from travel although we were unable to ascertain if infections were acquired during or prior to travel. All four patients had underlying comorbidities. Blood group-matched donors are accessed through community support groups from the hospital catchment area. MMC verified the COVID-19 history and current COVID-19 status of the donors with the respective medical care providers and through COVID-19 testing. The donors underwent routine screenings for blood donations and plasma was crossmatched.
Comorbidities among the four patients were as follows.
- •
Patient 1 developed symptoms on the 1st day of arrival; he was on regular medications for systemic hypertension and ischemic heart disease. He was tachypneic, and had fever and normal blood pressure on arrival.
- •
Patient 2, who was diabetic and hypertensive for the past 6 years, developed symptoms 4 days after a train journey.
- •
Patient 3 was diabetic and hypertensive, and developed low-grade fever the same day of travel.
- •
Patient 4, who was on treatment with sulfasalazine and low dose prednisolone for rheumatoid arthritis for the past 6 years, had a diagnosis of pulmonary tuberculosis 2 weeks prior to his travel. After arrival on June 19, 2020 he began antituberculosis treatment (isoniazid, rifampicin, ethambutol, and pyrazinamide) on the same day but experienced worsening of cough, fever, sore throat, and dyspnea for 3 days.
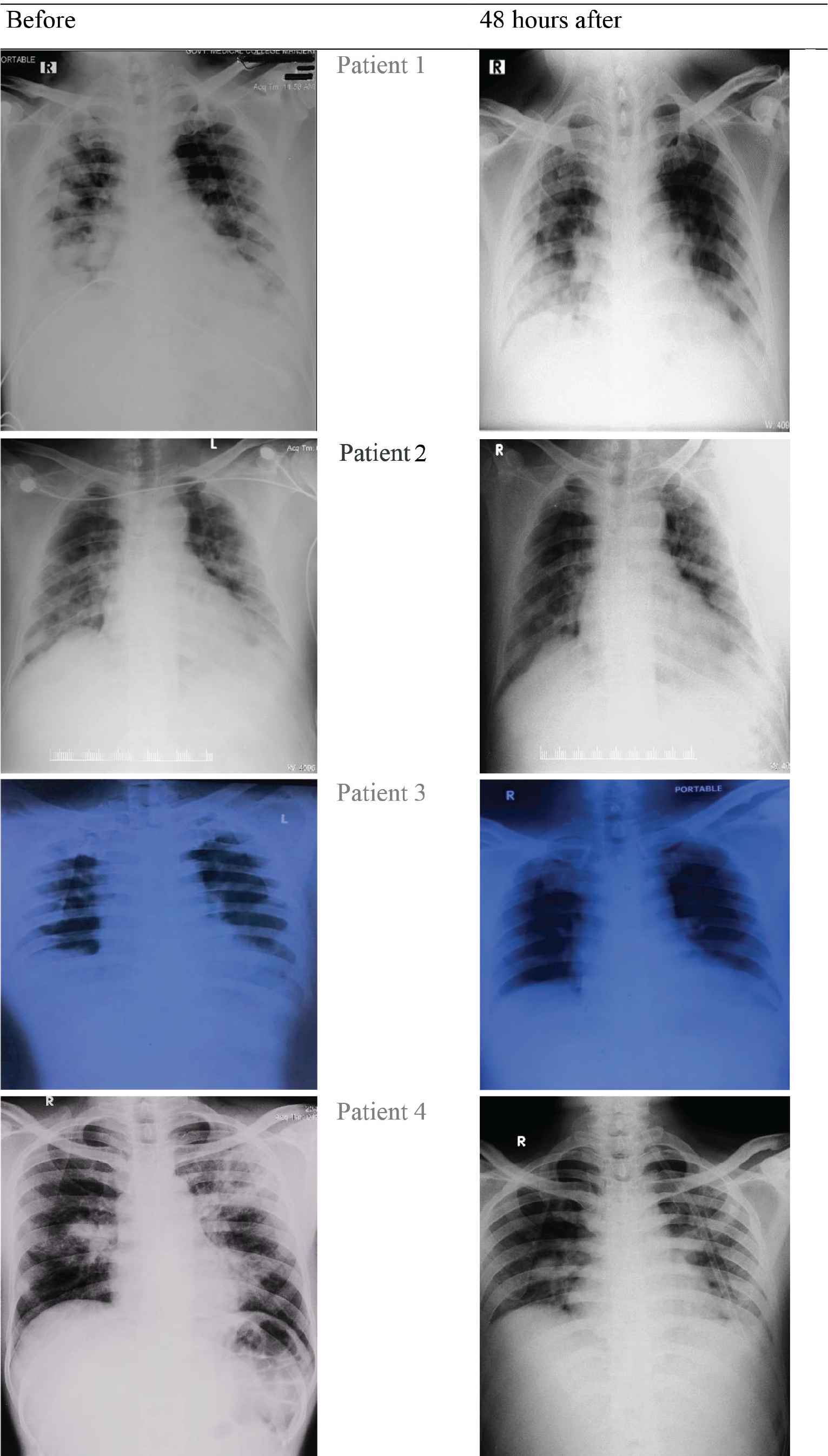
All four COVID-19 patients showed a favorable clinical course (Figure 1) after infusion of convalescent plasma drawn from patients who recovered from COVID-19 in the past 15–60 days, verified on chest X-rays (Appendix 1) and confirmed by COVID-19 Reverse Transcription-Polymerase Chain Reaction (RT-PCR) test.
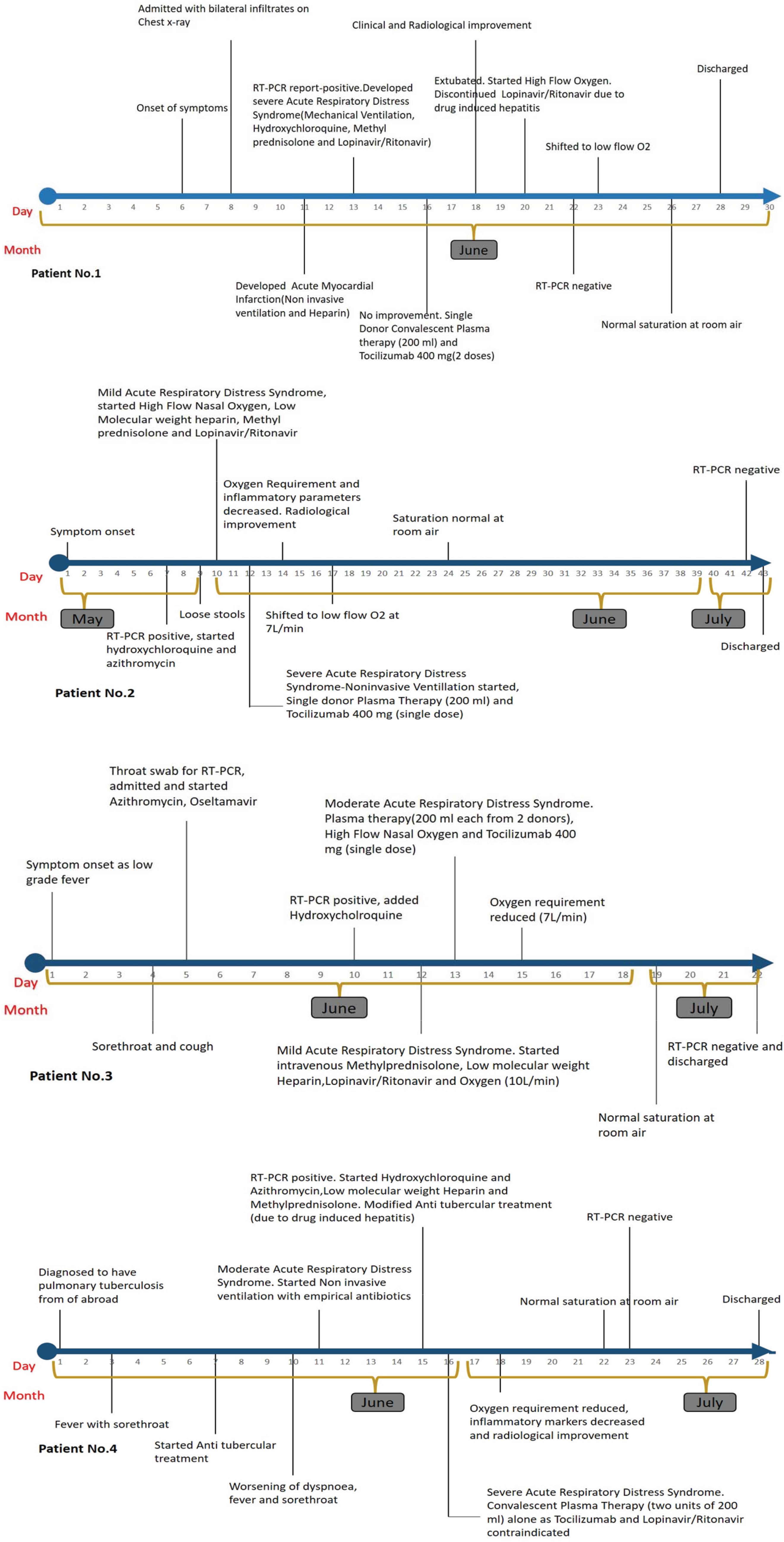
Clinical course of first four COVID-19 patients with ARDS who received convalescent plasma from COVID-19 survivors, Kerala State, India. HCQ, hydroxychloroquine
3. DISCUSSION
In this first series of four COVID-19 patients in Kerala, India, with comorbidities and deteriorating ARDS, the timely administration of convalescent plasma helped reverse the deterioration of severity within 48 h. The rationale for plasma therapy was deterioration of ARDS despite treatment with methyl prednisolone, lopinavir/ritonavir, hydroxychloroquine, and azithromycin. All four recovered fully from COVID-19 as demonstrated by SARS-CoV-2 PCR negative test results and were subsequently discharged. Among our four patients, improvement in patient clinical symptoms, requirement for oxygen, and overall well-being were observed within 24–48 h, but the number of days it took to decrease SARS-CoV-2 viral shedding as indicated by negative RT-PCR varied from 4 to 30 days of plasma therapy.
Our patients’ lack of improvement with lopinavir/ritonavir therapy may be attributable to their underlying comorbidities and also to the development of cytokine storm exhibited by hepatitis in one patient. Tocilizumab, an interleukin-6 inhibitor, is expected to ameliorate the inflammatory manifestations associated with severe COVID-19 and thus improve clinical outcomes [9]. Systemic corticosteroids may play a role in reducing excessive lung damage from inflammatory response to COVID-19 and reducing the risk of death among patients who develop ARDS [6–10]. However, corticosteroids are also known to suppress immunity through T-cell responses, reduce viral clearance, and delay antibody production [6,10]. Among patients with Middle East Respiratory Syndrome Coronavirus (MERS-CoV) admitted in Intensive Care Units (ICU) in 14 sites in Saudi Arabia during 2012–2015, corticosteroid therapy delayed the clearance of MERS-CoV RNA [9]. Similarly, corticosteroid therapy delayed viral clearance in avian influenza A (H7N9) and SARS [6–9,11].
Although this study did not assess the confounding effects of tocilizumab, our first report from a low-income country population adds evidence to findings from other countries on the utility of convalescent plasma in COVID-19 [9]. Informed by small single-center studies such as ours, subsequent large sample studies are being convened by the Indian Council of Medical Research (ICMR) to help inform the generalizability of our findings in the Indian context. ICMR’s multi-center clinical trial titled “A Phase II, Open-Label, Randomized Controlled Trial to Assess the Safety and Efficacy of Convalescent Plasma to Limit COVID-19 Associated Complications in Moderate Disease (PLACID Trial)” intends to recruit 452 patients from 21 centers.
Given the increasing scarcity of COVID-19 ICU beds in many countries, until more definitive therapeutic options become available, we posit that convalescent plasma therapy remains an option to reverse deterioration of critically ill COVID-19 patients.
CONFLICTS OF INTEREST
The authors declare they have no conflicts of interest.
AUTHORS’ CONTRIBUTION
All authors except SE are involved in clinical care of COVID-19 patients at MMC, and contributed equally to data collection. NK developed the first draft, MCA developed the graphical display, and SE coordinated the edits and comments and finalized the paper.
APPENDIX 1 Chest X-rays of four COVID-19 patients with acute respiratory distress syndrome before and after 48 hours of convalescent plasma therapy in Kerala, India
REFERENCES
Cite this article
TY - JOUR AU - Nisar Karekadavath AU - Shinas Babu Pullichola AU - E. Muhammed Afsal AU - Anitha S.P. Prabhu AU - Mansoor C. Abdulla AU - Shahul H. Ebrahim PY - 2020 DA - 2020/09/10 TI - Convalescent Plasma Therapy in First Four Critically Ill COVID-19 Patients in Kerala, India JO - Dr. Sulaiman Al Habib Medical Journal SP - 87 EP - 91 VL - 2 IS - 3 SN - 2590-3349 UR - https://doi.org/10.2991/dsahmj.k.200908.001 DO - 10.2991/dsahmj.k.200908.001 ID - Karekadavath2020 ER -
