History and Development of Autologous Stem Cell Transplantation for Acute Myeloid Leukemia

- DOI
- 10.2991/chi.k.210703.002How to use a DOI?
- Keywords
- Autologous stem cell transplantation; acute myelogenous leukemia; cryopreservation; review; history
- Abstract
This review describes the development of cryopreservation, the birth of autologous stem cell transplantation (ASCT) and its past and present use to consolidate adult patients with acute myelogenous leukemia (AML). It summarizes the first autografts in patients in relapse, the experience of autografting in complete remission (CR), using bone marrow unpurged or purged in vitro with cyclophosphamide-derivatives, and the important shift to peripheral blood stem cells. The review also discusses the results of recent studies in favor of the use of ASCT to consolidate good- and intermediate-risk patients who reach CR with no detectable minimal residual disease, and those which support the inclusion of maintenance therapy post autograft with hypomethylating agents, anti-BCL-2, and, possibly, in the future, anti AML chimeric antigen receptor-T cells. Carefully applied to well-selected patients, ASCT may regain interest, because of its simplicity, its reduced toxicity, lower non-relapse mortality and better quality of life.
- Copyright
- © 2021 International Academy for Clinical Hematology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
The real story of autologous stem cell transplantation (ASCT), in general, and ASCT for acute leukemia in particular, starts with the story of stem cell cryopreservation [1].
Although studies on the effect of cold on viability of cells go back a very long time with Reaumur (1736) and Spallanzani (1787) as pioneers, the real modern and fruitful impetus resulted from the devastating observations of the two atomic bomb drops on Hiroshima and Nagasaki (Japan) at the end of World War II. These generated in the scientific and medical community a frenzy to determine how to cryopreserve stem cells, and to perform allogeneic SCT (allo-SCT), both as preventive measures from lethal irradiation. This was the time when commercial advertising, especially in United States, promoted “nuclear bomb shelters in your garden”, and the time when the pioneers of allo-SCT with an identical sibling, were presenting their first results at international transplant meetings, namely Jean Dausset (Paris, France, Nobel Prize 1980), Donald Thomas (Seattle, USA, Nobel Prize 1990), Georges Santos (Baltimore, USA), Dirk van Bekkum (Leiden, the Netherlands), Georges Mathe (Villejuif, France), Jon van Rood (Leiden, the Netherlands).
Research on stem cell cryopreservation lasted about 10 years, before giving birth in 1976 to ASCT, which was, at that time, autologous bone marrow transplantation (ABMT). ASCT was evaluated as a means to administer the highest possible doses of chemotherapy/irradiation to obtain maximum tumor reduction with the maximum tolerated dose defined by all organ toxicities.
For approximately another 10 years, effort was put into increasing high-dose therapy modalities for the treatment of hematological malignancies and solid tumors. Various high-dose combination therapies were generated. Some copied the so-called conditioning regimen, built to prepare for an allogeneic transplant, such as cyclophosphamide + total body irradiation (TBI) or busulfan + cyclophosphamide (BUCY). Other protocols were more specifically adapted to a specific disease such as the BCNU, etoposide, cytosine-arabinoside, melphalan (BEAM) or the cyclophosphamide, BCNU, etoposide (CBV) for lymphomas.
For acute myelogenous leukemia (AML), ASCT has been a special challenge. When we performed the first ASCT in Hopital Saint-Antoine in Paris, in 1976, less than 10% of patients with AML could be allo-transplanted, since the age limit was 35 years old and the probability of having a genetically identical sibling was less than 25%. Applying high dose consolidation with ASCT covered therefore an unmet need, because it was available to every patient including those aged up to 70 years, with slightly reduced-dose regimens, such as the BCNU, ARA-C, amsacrine, etoposide (BAVC) pioneered in Roma. Severe criticism came from everywhere, in view of the risk of reinfusing leukemic stem cells with the autograft (even though this was collected in CR). For about 15 years, the interest was focused on in vitro purging of the collected marrow from residual leukemic cells.
About 50,000 ASCT for acute leukemias (AML and acute lymphocytic leukemia) have been performed so far, most of them before the year 2000. ASCT for AML has benefitted from a very low non-relapse mortality (NRM) but suffered from a high relapse incidence (RI). In contrast, allogeneic transplantation’s graft-versus-leukemia (GVL) immunological effect has endowed it with a much lower RI, but an increased NRM.
In the last two decades, many major improvements have facilitated allo-transplants, most notably the possibility of finding a donor for almost every transplant eligible patient and the use of reduced intensity conditioning. Today, ASCT is widely used for the treatment of lymphoid malignancies. The number of ASCT performed in Europe each year in AML patients, as recorded by the European Society for Blood and Marrow Transplantation (EBMT), is fewer than 300.
Yet the story of ASCT may not be over. The management of AML has been greatly improved in the past decade. We now have a better definition of AML risk groups by combining cytogenetics with molecular markers. We can better evaluate minimal residual disease (MRD) and identify good quality remissions with undetectable MRD (negative) by flow cytometry and/or molecular biology. In several AML subtypes (FLT3-ITD or mutated, IDH1 and 2), we now have targeted therapies. We also have new agents such as hypomethylating agents (decitabine/5-azacytidine), BCL-2 inhibitors e.g. venetoclax, and new monoclonal antibodies such as anti-CD47 (magrolimab). Most of all, the introduction of maintenance therapy post-transplant, which was inconceivable even a few years ago, is now being evaluated by randomized studies.
Taking these improvements into account, recent retrospective studies of ASCT in AML indicate that there is indeed a population of patients that would benefit from ASCT rather than allogeneic transplantation.
2. CRYOPRESERVATION OF STEM CELLS
The danger of TBI leading to fatal bone marrow (BM) aplasia, as observed following the atom bomb explosions in Hiroshima and Nagasaki in 1945 led to speeding up of research in stem cell cryopreservation, and numerous animal models were developed immediately after World War II. It is noteworthy that the demonstration in 1955, by Barnes and Loutit [2], that BM could be successfully cryopreserved (after the initial successful work on preservation of bull sperm by Polge et al. [3] in 1949) did not allude to the preservation of marrow stem cells but rather, to the preservation of a so called “radiation recovery factor”.
Several preclinical models in mice [4], rabbits [5], monkeys [6,7] and dogs [8–13] demonstrated the ability of frozen marrow to engraft and reconstitute hematopoiesis. In 1975, we developed a canine in vivo model [8,10] to assess the viability of stem cells frozen and stored for prolonged periods in liquid nitrogen. We used a freezing scheme (Figure 1) in which BM suspended in tissue culture medium (M199) with a final 10% dimethyl sulfoxide (DMSO) concentration was refrigerated at a fixed cooling rate of −1°C/min [8] with permananent temperature recording to detect the release of the heat of fusion and supercool at this very moment by massive introduction of liquid nitrogen. The rationale was to avoid the destruction of cells due to the reorganization of extra-cellular ice induced by this release, similar in a way, to a ship imprisoned in a melting and re-icing floe. Forty-six foxhounds received TBI (10 Gy) followed by the infusion of autologous fresh BM or frozen BM stored for 2–5 months in the vapor phase of liquid nitrogen (−140°C). The results demonstrated a direct relationship between the dose of BM infused and the percentage of successful engraftments. The minimum dose of fresh BM for autologous engraftment was between 0.1 and 0.25 × 108 nucleated cells/kg. There was no difference between fresh BM and BM stored for 2 months (100% recovery of frozen stem cells) (Figure 2A). Parallel in vitro studies showed destruction of mature myeloid elements but perfect ultrastructure of frozen and thawed lymphocytes, plasma cells and erythroblasts (Figure 2B). Also, frozen, and thawed marrow grown in short-term culture produced normal metaphases, with no chromosome aberrations. DNA synthesis evaluated in 106 nucleated cells before and after preservation was similar. In 1975, the medical scene for ABMT was set.
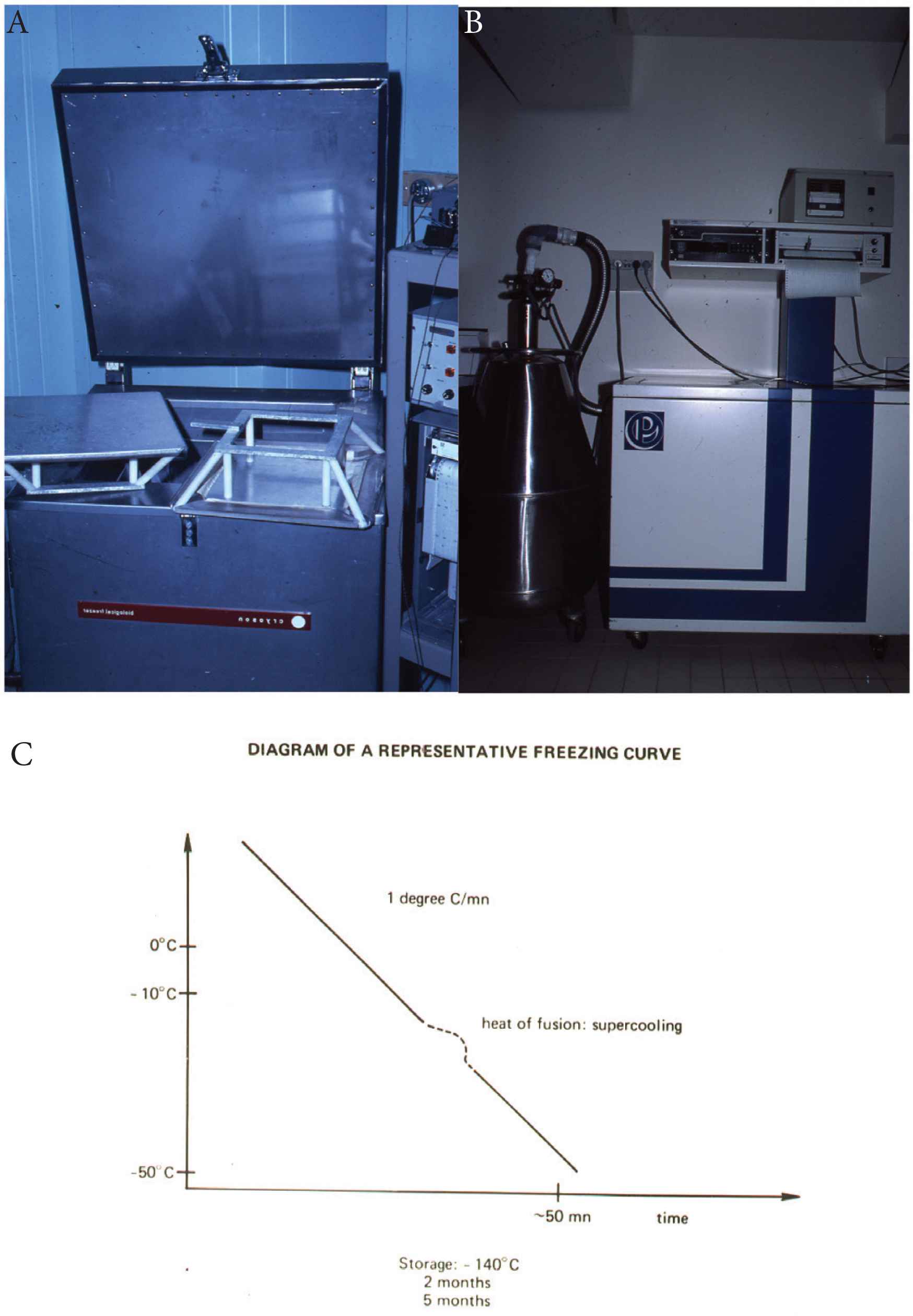
The first programmed freezing apparatus. (A) « Cryoson » (Midden Beemster, The Netherlands) used at Hôpital Saint-Antoine, Paris, from 1974 to 1995. (B) Nicool, the freezing apparatus from “Air liquid” used afterward. (C) The ideal freezing curve with a −1°C/min constant cooling rate and supercooling at the release of the heat of fusion to avoid thermic shock.
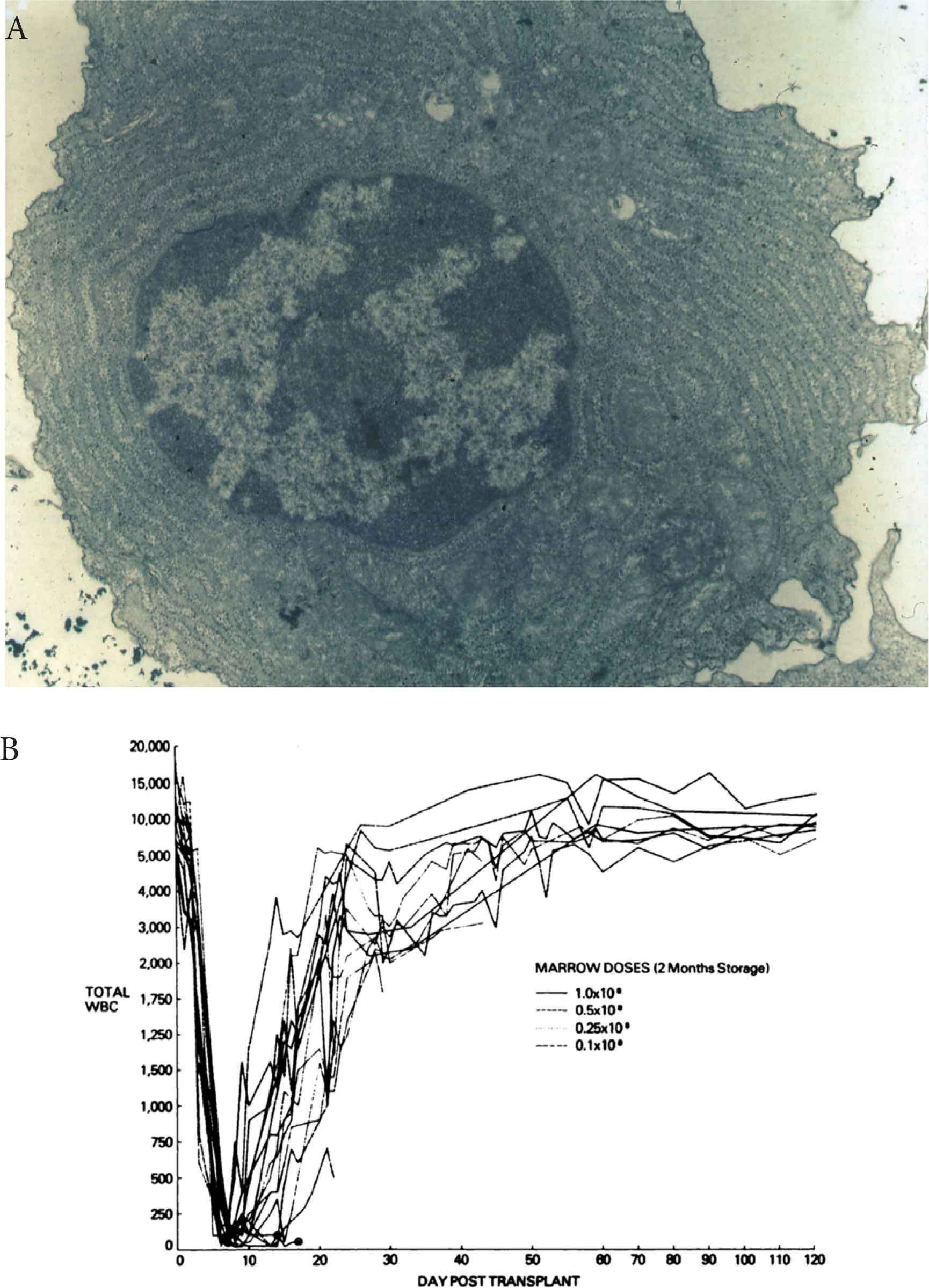
The preclinical canine model. (A) A well cryopreserved plasmocyte with no ergastoplasm disruption. (B) Composite of total leukocyte counts for dogs submitted to total body irradiation followed by infusion of various doses of frozen autologous marrow stored for 2 months.
We later showed, in humans, that over 75% of the proliferative capacity of the cryopreserved granulocyte-macrophage colony-forming unit (CFU-GM) progenitors was recovered on thawing, but that DMSO caused substantial loss of progenitor cells within 20 min at 4°C [14] and that the recovery of CFU-GM from cryopreserved marrow was predictive of engraftment [15]. We therefore decided to freeze in parallel to the stem cell graft itself, a small control probe sample, which could be tested before the autograft infusion. This was especially useful after a long storage duration or uncertainty about a possible breaking of the cold chain [16].
Theoretically, stem cells stored at −140°C in the gas phase of liquid nitrogen or even deeper at −196°C can be preserved indefinitely, since all enzymatic processes are totally blocked, which is not the case at lower temperatures of −20°C or even −70°C. Safe preservation for periods as long as 11 years have been reported [17].
Many teams have tried to simplify the cryopreservation technique or to replace it by storage of the harvested marrow in a conventional refrigerator at 4°C. However, it was shown very early on that marrow could not be preserved for more than 56 h [18]. In contrast, and more recently, it was observed that peripheral blood stem cell (PBSC) collected and somewhat purified by leukapharesis (LK), can survive storage at 4°C for up to 6 days but not longer, enabling carefully planned autografts to be performed in patients receiving short pre-transplant regimens [19–24]. Stem cell cryopreservation opened the door for autologous transplantation and a decade later to cord blood storage.
3. HISTORICAL AUTOLOGOUS STEM CELL TRANSPLANTATION FOR ACUTE MYELOID LEUKEMIA
3.1. The Premise
Following our pioneering work on stem cell cryopreservation [8–10,25] with the demonstration of its efficacy in a preclinical dog model, we performed at hopital Saint-Antoine, in Paris in February 1977, the first ABMT in a 28-year old male patient with AML [26,27] (Figure 3). The patient had gone into an early drug resistant relapse with multiple chloromas (one of them on an upper eyelid leading to a complete obstruction of the field of vision), while on maintenance chemotherapy with methotrexate and 6-mercaptopurine, 7 months after the induction of a first CR (CR1). He received a myeloablative regimen followed by marrow collected while in early remission and cryopreserved in the gas phase of liquid nitrogen. The patient experienced a second CR (CR2) of short duration. A second patient experienced a CR2 which lasted 4 years before relapsing. From these initial observations until 1982, we and several other teams followed this approach and demonstrated that in adult patients with AML in relapse, a high rate of CR could be achieved, which for the majority, lasted longer than the initial CR. The term “inversion” was used at that time to draw attention to such an unusual evolution contrasting with the so called “natural” evolution of the disease. ABMT was tested in parallel in patients with refractory and relapsing lymphomas and myelomas and it was observed that the kinetics of hematopoietic (neutrophils and platelets) recovery in AML were much longer than in lymphoid malignancies [26–29].
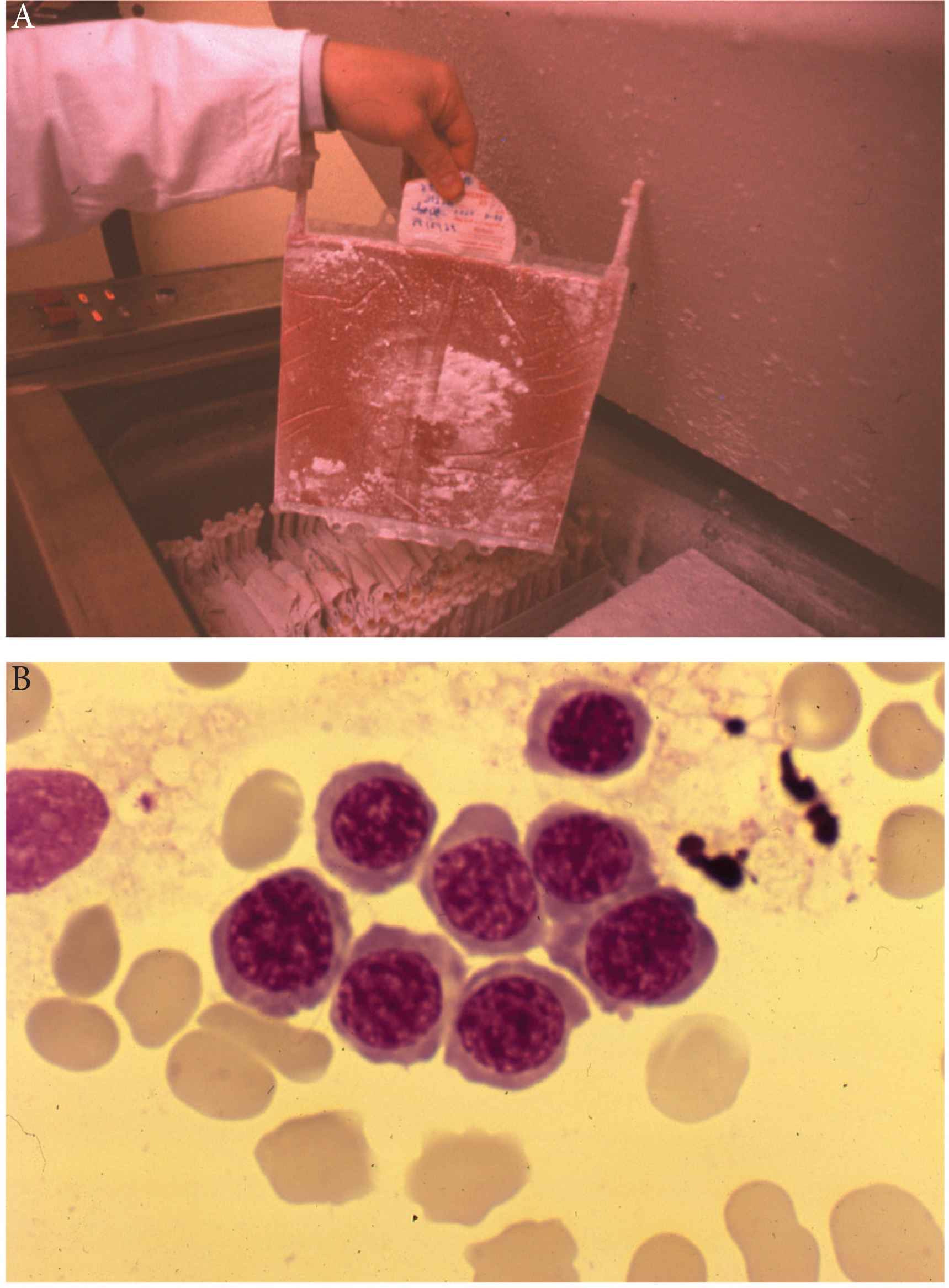
The first autologous stem cell transplantation in man (1976). (A) A polyolefin bag containing marrow of one of the first autografted patients, squeezed between the two plates presented on Figure 1 and frozen following a program of −1°C/min with abrogation of the heat of fusion. Polyolefin bags were fragile and could break on thawing. They were later replaced by Teflon–Kapton bags. (B) Marrow aspirate 14 days after the first patient was autografted at Hopital Saint-Antoine, Paris. The patient received his marrow collected in first remission of acute myelogenous leukemia and cryopreserved in the gas phase of liquid nitrogen (−140°C) in 1976: the first erythroblastic nest attesting for engraftment.
3.2. Autologous Bone Marrow Transplantation with Purged and Unpurged Marrow
From 1982, the use of high dose myeloablative therapy as consolidation with ABMT was applied to patients in CR. However, concerns were raised about the risk of reinfusing leukemic stem cells with the autograft usually harvested in early CR1. A minimum number of consolidation chemotherapy courses preceding marrow harvesting was found to be beneficial to minimize this risk and referred to as “in vivo purging”. Also, and even more importantly, techniques of in vitro purging of the autograft were developed prior to cryopreservation and reinfusion. The Baltimore team showed that 4-hydroperoxy cyclophosphamide (4HC), a direct acting cyclophosphamide catabolite, could selectively destroy in vitro leukemic stem cells, while sparing normal stem cells protected by a high level of aldehyde dehydrogenase. In a highly demonstrative experiment, Sharkis et al. [30] prepared cell suspensions of normal rat marrow mixed with rat acute myelogenous leukemia cells which they incubated in vitro with graded doses of 4HC. The cell suspensions were injected into syngeneic rats prepared with a lethal dose of TBI. Animals injected with these cells survived fatal irradiation induced aplasia. Animals given cell suspensions incubated with the lower doses of 4HC showed prolonged survival before death from leukemia while animals given cell suspensions incubated with higher doses of 4HC survived lethal irradiation and were cured of leukemia. These studies clearly established that tumor cells may be eliminated from normal marrow suspensions without completely destroying the pluripotent stem cells. Several teams throughout the world, in the United States [31], France [32], including our team in Paris [33–36], Germany [37] and Italy started programs of ABMT with high dose cyclophosphamide and TBI (single dose at 10 Gy or fractionated at 12 Gy) using marrow purged in vitro with either 4HC in the United States, or mafosfamide (a related compound) in Europe. Most teams treated the marrow with fixed doses of mafosfamide while we adjusted the dose to what we considered to be the maximum tolerated dose defined as sparing 5% residual CFU-GM [33,35,36]. Interesting observations resulted from this approach.
On technical grounds, it was confirmed that in AML, but not in lymphoid malignancies, marrow and purging with mafosfamide resulted in delayed kinetics of recovery of hematopoiesis; we observed engraftment of neutrophils by day 30 only (range, 12–153), and platelets by day 90 only (range, 19–850) [34]. Various chromosome abnormalities including in chromosome 1 were detected years after the autograft, with no relation to the initial leukemia and no detectable impact on outcome. Whether they resulted from mafosfamide treatment or previous TBI given with the pre-transplant regimen, could not be determined [38,39]. Interestingly, it was observed that clonogenic leukemic progenitor cells in AML are highly sensitive to cryopreservation itself (six different methods were tested) which therefore contributed to purging in addition to mafosfamide [40].
Several clinical studies supported this approach. Our team at Hopital Saint-Antoine [41] reported on a total of 229 consecutive patients autografted in CR with marrow purged with mafosfamide. The patients receiving the highest stem cell doses evaluated before purging and the most aggressively treated with mafosfamide, as evaluated by the fraction of residual CFU-GM, had a treatment-related mortality of only 5 ±2%, a leukemia-free survival (LFS) of 70%, and an overall survival (OS) of 77 ± 7% at 10 years post transplantation.
The EBMT published two retrospective studies in 1990 and 1992 [42,43] comparing the outcome of AML patients autografted while in CR with marrow, purged or unpurged. The second report involved 59 European teams that had reported 919 autografts for consolidation of AML up to December 31, 1989. Marrow was purged with mafosfamide in 269 patients. Multivariate analysis showed significant efficacy of marrow purging in AML in CR1. In patients autografted after TBI, the RI with purged marrow was 29% versus 50% with unpurged marrow, and 16% versus 60% when considering only those autografted within 6 months of CR. In slow responders, the results were 20% versus 61%, significantly in favor of purging, whereas the RI were similar in rapid responders. The relapse patterns were different in that the plateau for persisting remission started at 23 months with purged marrow and at 32 months with unpurged marrow. It was concluded that purging was most likely to bring benefit to a specific category of patients, i.e. those transplanted early, and slow responders, in whom the probability that leukemic cells might still persist in the graft at the time of collection was higher. These data were confirmed 9 years later by an International Bone Marrow Transplant Registry (IBMTR) retrospective study on patients with AML autografted in the United States [44]. In this latter study of 294 patients, multivariate analysis showed that patients receiving 4HC-purged transplants had a lower relative risk (RR) of treatment failure than those receiving unpurged transplants (RR, 0.69, p = 0.12) in the first post-transplant year and thereafter (RR, 0.28, p < 0.0001). Adjusted 3-year probabilities of LFS, were 56% and 31% after 4HC-purged and unpurged transplants in CR1, respectively. Corresponding probabilities in CR2 were 39% (25–53%) and 10% (1–29%). The authors concluded that grafts purged with 4HC were associated with higher LFS after ABMT for AML.
In 1992 Malcolm Brenner [45,46], using gene marking studies, demonstrated that leukemic cells harvested with an autologous graft could contribute to relapse. The neomycin resistance gene in a retroviral vector was used to mark autologous unpurged marrow infused into patients; in the few patients who relapsed, the resurgent blast cells contained the neomycin-resistance gene marker, clearly indicating that they originated from the graft. Similar observations were made with neuroblastomas, chronic myelocytic leukemia, and solid tumors. It was felt at that time that the proof of principle was strongly established to support routine in vitro purging. However even though the technique of marrow purging was relatively simple, it was not available in most centers. From 1994, peripheral blood (PB) became the major source of stem cells and using 4HC or mafosfamide to treat large volumes was more complex. Centers pursuing their programs of high-dose consolidation with autografting in AML in CR shifted to PBSC with no in vitro purging.
3.3. The Shift to Autologous Peripheral Blood Stem Cell Transplantation
The use of PBSC to autograft patients with AML was an important turning point. It benefitted from all the technical advantages of PB over marrow collection (simplicity, lower risk, no anesthesia or operating room, and easier planification of LK). Unfortunately for AML patients, the rapid shift to PB probably resulted in an increase in RI. Early experiences with PBSC transplants were disappointing and, in fact, suggested that at least if mobilization and collection followed immediately after induction, a large proportion of leukemic clones were mobilized. Massive reinfusion of these cells into patients led to early relapses [47,48]. Leukapheresis after several consolidation courses for “in vivo purging” was then recommended and it was shown in a French randomized, multicenter study that patients undergoing LK after a minimum of two chemotherapy courses had a trend toward a lower RI (47% versus 57%, p = 0.1) and a better LFS (48% versus 39%, p = 0.1) than patients undergoing LK after only one chemotherapy course [49].
A first EBMT retrospective study between BM (purged and unpurged) and PB as sources of stem cells for autografting suggested better results with BM [50]: in this comparison, 1393 patients undergoing either a PBSC (n = 100) or purged (n = 252) or unpurged (n = 1041) BMT were compared. Hematopoietic recovery was significantly quicker after PBSC than after either purged or unpurged BMT. The 2-year LFS, RI and OS for the entire study population was 52%, 43% and 58%. After PBSC transplants, LFS and RI were 44 ± 6% and 50 ± 6% and did not differ significantly from that found for unpurged BM transplants (49 ± 2% and 45 ± 2%; p = NS). However, LFS (57 ± 3%) and RI (37 ± 3%) of patients undergoing purged BMTs were significantly better than that found for PBSC patients (p = 0.01 and 0.006, respectively). As some characteristics of patients undergoing PB or purged BMT differed, the better outcome observed for purged BM over PB patients could not be firmly established.
In a subsequent EBMT retrospective study [51], the Acute Leukemia Working Party analyzed 2165 patients who received autografts (1607 PBSC and 558 BM) from 1994 to 2006. Relative to the time of CR1, PBSC transplants were performed earlier than BM transplants. Because a poorer outcome was associated with a shorter interval from CR1 to transplantation, patients were divided into three transplant groups: BM, early PBSC (≤80 days after CR1), and late PBSC (>80 days after CR1). In a multivariate analysis adjusted for differences between groups and centers, RI was higher with both early PBSC (56 ± 3%; p = 0.006) and late PBSC transplantation (46 ± 2%; p = 0.01) as compared with BMT (39 ± 2%). This translated into a significantly worse LFS for early PBSC transplantation (36 ± 3%; p = 0.02) and a trend towards a poorer LFS for late PBSC (46 ± 2%; p = 0.06) as compared with BMT (52 ± 2%). It was concluded that for patients with AML in CR1, the risk of relapse was greater with PBSC transplantation than with BMT.
Indirect evidence that mobilization of stem cells also mobilized residual leukemic progenitors resulted from the observation first by the European Organization for Research and Treatment of Cancer (EORTC) [52] as early as 2003 and later confirmed by the EBMT that patients autografted with the highest dose of CD34(+) cells obtained with LK had a higher RI and a lower LFS: the EORTC concluded that a high percentage of CD34(+) cells in autologous AML PBSC products reflected inadequate in vivo purging in a subgroup of patients with poor clinical outcome. In an EBMT study [51] of 772 patients autografted more than 80 days after CR1, the highest quintile for CD34(+) stem cell dose infused (>7.16 × 106/kg) was selected as the cut-off point. Relapse was more frequent in patients who received the highest dose (HR = 1.48; p = 0.005), and LFS was worse (HR = 0.72; p = 0.01).
A more recent study of 956 patients (which led to the use of a nomogram for Individualized Prediction of LFS after ASCT) also found that BM was associated with better outcomes than PBSC [53] but stem cell source was removed from the score calculation because of the possibility of associated confounding factors.
A randomized study comparing autologous BMT versus autologous PBSC has never been done and unfortunately is unlikely, due to the collection of mobilized PBSC being much easier and more importantly, the kinetics of engraftment being much shorter than with BM, resulting in a reduction of the duration of aplasia and NRM.
Currently, for these practical reasons, the established routine is to use PBSC for autografting AML patients. However, in view of previous experience, a minimum of two high-dose chemotherapy consolidation courses is recommended before LK as well as careful monitoring of MRD by either flow cytometry and/or molecular biology so that mobilization is carried out on patients with no detectable MRD. The verification of the absence of detectable residual disease in the collected PBSCs (the autograft itself), is an additional safety measure.
3.4. Retrospective Studies with Bone Marrow
From 1976 to 1995, reports of ASCT in AML concerned single institutions or data from registries. The situation was similar for allo-transplants.
Results from these reports are summarized in a previous review [54]: For patients autografted with unpurged marrow, the LFS ranged from 34% (Sutton, UK) to 70% (Bologna, Italy) at 5 years with a median of 50%. With purged marrow, LFS ranged from 41% (Baltimore, USA) to 80% (Manchester, UK); our own series with cyclophosphamide + TBI and purging with adjusted doses of mafosfamide on 64 patients had a LFS of 58% and a RI of 25%. A similar series of 50 consecutive patients autografted in San Francisco with marrow purged by 4HC was reported with an LFS that plateaued at 70% and a RI of 27% with a median follow up of 7 years [55,56]. In CR2, the two largest series came from Baltimore (purging with 4HC) with a LFS of 30% in 80 patients, and from Rome (no in vitro purge) in 60 patients who received the specific BAVC pre-transplant regimen with a LFS of 42% at 10 years [57].
The general assumption during this period was that ABMT with no in vitro purging, as consolidation for all consecutive adult AML patients, with no stratification for cytogenetics, resulted in a 50% LFS in CR1 and about 30% in CR2. ABMT with in vitro purging was credited with better outcome but no comparison was possible.
As can be easily seen from these results the major drawback was a high RI.
Interest in single institution reports disappeared when results from randomized studies became available.
3.5. Randomized Studies in CR1 with Bone Marrow or PBSC
In 1995, Zittoun et al. [58] published the first phase 3 randomized study comparing the value of high dose consolidation followed by ABMT. Patients with AML in CR1 and with an HLA-identical sibling were assigned to undergo allogeneic BMT; the others were randomly assigned to undergo ABMT (with unpurged bone marrow) or a second course of intensive chemotherapy, combining high-dose cytarabine and daunorubicin. The projected rate of disease-free survival (DFS) at 4 years was 55% for allogeneic transplantation, 48% for autologous transplantation, and 30% for intensive chemotherapy. Interestingly these figures matched the results observed at that time from EBMT retrospective registry studies. However, the OS after CR was similar in the three groups, since more patients who relapsed after a second course of intensive chemotherapy had a response to subsequent ABMT. They concluded that autologous as well as allogeneic BMT resulted in better LFS than intensive consolidation chemotherapy.
These results were confirmed in 1998 by the UK MRC AML10 [59] study which randomized 381 patients to either intensive chemotherapy or ASCT. Of the 190 patients allocated to autologous BMT, 126 received it. On intention-to-treat analysis the number of relapses was substantially lower in the autologous BM group than in the group assigned to no further treatment (37% versus 58%, p = 0.0007), resulting in superior LFS at 7 years (53% versus 40%; p = 0.04). These benefits were observed in all risk groups and age groups. There were more deaths in remission in the autologous BM group than in the no further treatment group (12% versus 4%, p = 0008). There was a trend for an OS advantage in the autologous BM transplant group at 7 years (57% versus 45%, p = 0.2).
In 1998, the French GOELAM group [60] randomized 367 patients who reached CR1 from an initial population of 517 patients up to 40 years of age, to receive either an allogeneic transplant with an HLA-identical sibling (if available) or after a first course of consolidation with high-dose cytarabine and anthracycline, to randomly receive a second course of consolidation with amsacrine and etoposide or a combination of busulfan and cyclosphosphamide, followed by an unpurged autologous BMT. There was no difference in outcome in the three groups. The absence of superiority of allo-SCT which was unexpected, was difficult to explain.
Similarly, in 1998, the ECOG/SWOG/CALGB US intergroup [61] reported the results of a comparison of ABMT (purged with 4HC) versus allo-SCT versus high dose ARA-C. In an intention-to-treat analysis, they found no significant differences in LFS among patients receiving high-dose chemotherapy, those undergoing ABMT, and those undergoing allogeneic BMT. These data were also a subject of high controversy. Indeed, when they were reanalyzed using cytogenetics [62], the conclusion appeared somewhat different: it was found that patients with favorable cytogenetics did significantly better following ABMT and allogeneic BMT than chemotherapy alone, whereas patients with unfavorable cytogenetics did better with allogeneic BMT. Patients in the intermediate-risk cytogenetics group did equally well in the three arms.
Several other randomized studies have been reported. In the last meta-analysis [63] published in 2004, 1044 patients from six eligible studies were randomly assigned to receive ABMT or non-myeloablative chemotherapy (five studies) or ABMT or no further treatment (one study). Compared with patients who received chemotherapy or no further treatment, patients who received ABMT had a better LFS (p = 0.006) but a similar OS. The authors concluded that the results did not support the routine use of autologous transplantation in adult AML in CR1. This can be challenged however, by the fact that NRM at that time was higher than at present, and also by the fact that reducing the relapse rate has often preceded any demonstration of a benefit in survival.
4. AUTOLOGOUS STEM CELL TRANSPLANTATION FOR ACUTE MYELOID LEUKEMIA NOWADAYS
4.1. Recent Developments
The considerable progress in allo-SCT which now makes it possible to transplant almost any AML patient, has resulted in a sharp decrease in the numbers of ASCT done each year for AML. Nonetheless, some advances in the field of ASCT have occurred regarding the optimization of the pre-transplant regimen and better selection of patients and disease status at transplant, to obtain a better outcome. In addition, the development of various maintenance therapies to reduce the risk of relapse post ASCT, including targeted drug therapies and immune therapies such as chimeric antigen receptor (CAR)-T cells have brought hope. Today, one option is to offer ASCT as an alternative to allo-SCT to good- and intermediate-risk patients in CR1 with no detectable MRD and thereby obtain similar LFS and OS with no risk of graft-versus-host disease (GVHD), mostly severe chronic GVHD and a better quality of life (QoL).
4.2. A More Effective Myeloablative Pre-transplant Regimen
The combination of cyclophosphamide (CY) (60 mg/kg/day) + fractionated TBI (200 cGY morning and afternoon for 3 days) separated by a 2-day interval (either CY-FTBI or FTBI-CY) [64] or the combination BUCY [31,65,66] (Busulfan 4 mg/kg/day for 4 days followed by CY 60 mg/kg/day for 2 days) have been the historical myeloablative regimen pre-autograft. A specific reduced toxicity regimen, the busulfan, amsacrine, etoposide, cytosine-arabinoside (BAVC) was developed in Rome [57] and was well tolerated in older patients.
Other regimens including etoposide were tested with promising results: the Stanford team for example, combined high-dose etoposide with busulfan (BU-VP16) [67] and the Japanese [68] and Spanish Pethema group used a combination of busulfan, etoposide and cytosine-arabinoside.
Two recent improvements are of importance. Firstly, the shift from oral busulfan to intravenous (IV) administration. A recent retrospective analysis from the EBMT [69] has suggested a better LFS in AML patients in CR1 which is even more impressive in CR2: we analyzed data from 952 patients with AML who received intravenous busulfan for ASCT. Two-year OS, LFS, and RI were 67 ± 2%, 53 ± 2%, and 40 ± 2%, respectively. The NRM rate at 2 years was 7 ± 1%. Overall LFS and RI at 2 years did not differ significantly between the 815 patients transplanted in CR1 (52 ± 2% and 40 ± 2%, respectively) and the 137 patients transplanted in CR2 (58 ± 5% and 35 ± 5%, respectively).
Secondly, the finding that the combination of IV busulfan and high-dose melphalan (BUMEL) was associated with the best OS (75 ± 4%), a finding that was in accordance with previous Italian studies from the Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto (GIMEMA) group [70] which had already drawn attention to BUMEL.
The EBMT therefore reevaluated the various conditioning regimens [71,72] and confirmed the superiority of BUMEL over BUCY in a first limited series of 853 patients autografted in CR1. In a subsequent analysis, to identify the subpopulations that might best benefit from BUMEL, all adult patients with primary AML and with available cytogenetics, autografted from January 2000 to December 2016 in CR1 (1137 patients who received BUCY and 512 BUMEL) were evaluated in depth. In the poor-risk group defined as poor cytogenetics and/or presence of the FLT3-ITD mutation, BUMEL was associated with a lower RI at 5 years (53% versus 69%; p = 0.002), a better LFS (42% versus 25%; p = 0.002) and a better OS (54% versus 36%; p = 0.02). In the non-poor-risk group, there was no significant difference. The conclusion was that BUMEL is a more potent antileukemic combination and is the preferable conditioning regimen for the poor-risk leukemic patient, while in AML patients without poor-risk cytogenetics or FLT3 mutation, BUCY may remain a valid option.
Therefore, today the combination of IV busulfan and melphalan is our recommended pre-autografting regimen for adult AML in CR (Figure 4).
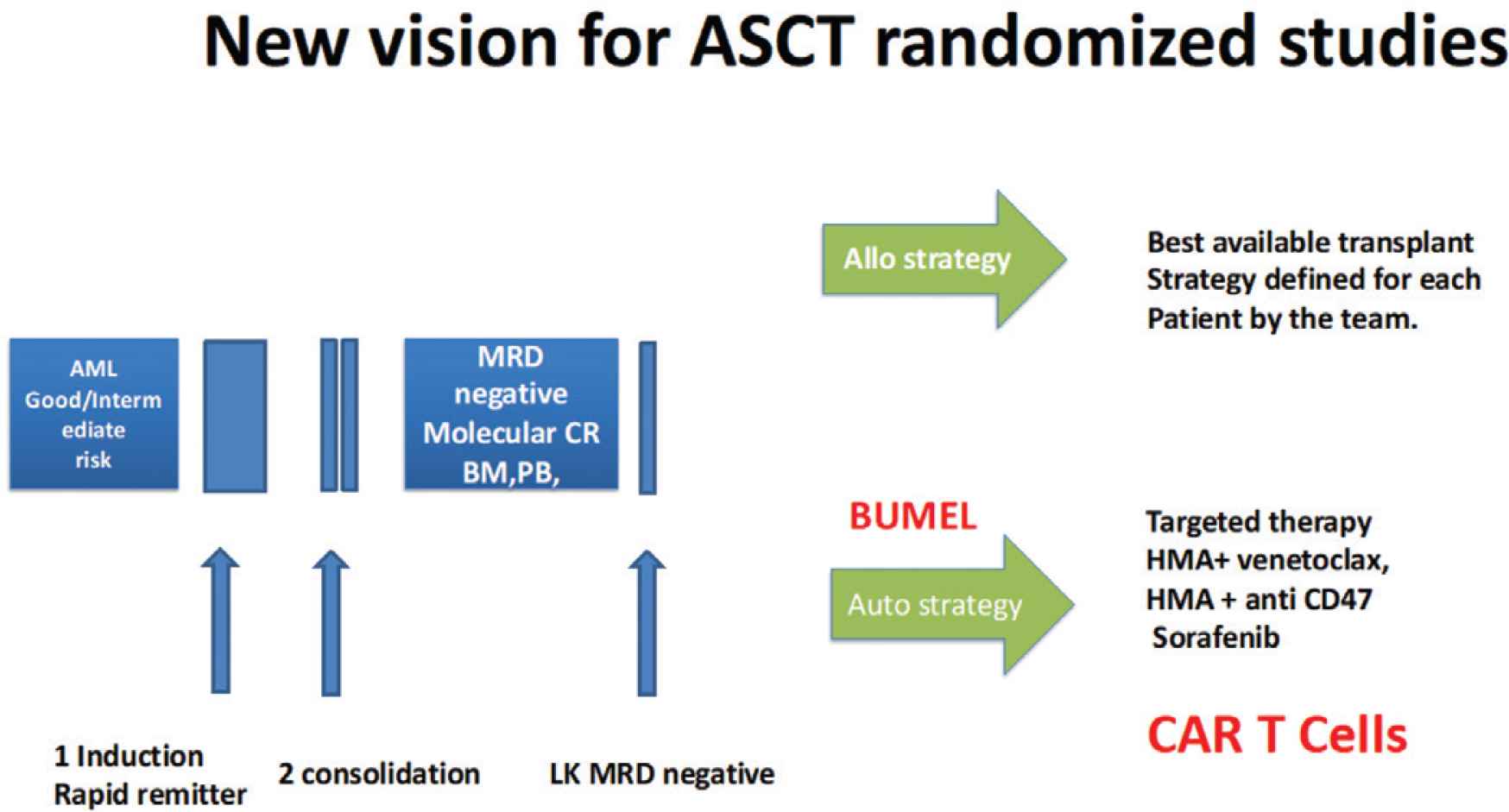
One possible scheme of a modern randomized study reassessing ASCT for the consolidation of patients with AML in MRD negative CR.
4.3. A Better Selection of Patients
Allogeneic stem cell transplantation has at first sight several advantages over ASCT for the curative treatment of adult AML: The first is the existence of the GVL effect; a second is the use in many instances (although not always) of reduced intensity conditioning; a third advantage is the recent possibility of using haploidentical donors as an alternative stem cell source which, combined with the other available sources, now makes it possible to offer an allo-transplant to almost any patient with AML who needs one.
In contrast, depending upon the patient population and the status of the disease, allo-transplantation has a high NRM rate in the range of 15–25% and an incidence of severe chronic GVHD of approximately 10%. For this reason, the outcome is nowadays expressed not only in terms of LFS and OS but also as the composite endpoint of GVHD-free, and relapse-free survival [73].
Autologous stem cell transplantation has some drawbacks, primarily the necessity to use a myeloablative regimen pre-transplant and a higher RI post-transplant. In contrast, it benefits from a much lower NRM and in the absence of GVHD a better QoL post-transplant [74–76]. Early attempts at generating GVHD/GVL post ASCT with interleukin-2 [77] or Cyclosporin A although promising, have failed [78] but they may have paved the way for the introduction of immune therapies (see below).
In view of these opposite advantages and inconvenience, the question that has been raised in the past decade is whether one can define a population of patients that may be cured of AML using ASCT, with a similar rate to allo-transplant, taking advantage of a lower NRM and resulting in a better QoL. Indeed, several retrospective studies have pointed out that adult AML patients in CR with no detectable residual tumor (MRD negative) using either flow cytometry and/or molecular biology may indeed achieve results similar to allo-transplantation. The present development of targeted therapies including immune therapies that might be given post ASCT further supports this approach.
Analyses from many registries have shown that ASCT benefits essentially AML patients classified as good- and intermediate-risk by cytogenetics, and not poor-risk patients in whom an allo-transplant is the only potential curative approach: The Italian Group for Blood and Marrow Transplantation reported on a total of 809 patients autografted in CR1 [79] with 2-year LFS rates of 64 ± 8%, 48 ± 4% and 46 ± 7% in good-, intermediate-, and poor-risk patients, respectively (p < 0.0001), while the 2-year OS rates were 79 ± 7%, 63 ± 4% and 59 ± 8%, respectively. A multicenter retrospective study in Seoul (Korea) [80] revealed that younger patients aged <40 years old), with good- to intermediate-risk molecular cytogenetics, and who received limited doses of CD34(+) stem cells might be good candidates for ASCT with a 3-year LFS and RI of 83% and 17%, respectively. Mizutani et al. [81] using the Japanese registry retrospectively compared the outcomes of patients who underwent autologous PBSC transplantation in CR1 (ASCT, n = 375) with those who underwent allogeneic BMT (n = 521) and allo-PBSC (n = 380) from a matched sibling donor. The LFS of ASCT was not significantly different from that of allo-BMT (HR: 1.23; p = 0.16) and allo-PBSC (HR, 1.13; p = 0.40). They concluded that auto-PBSC transplantation remains a promising alternative treatment for patients with AML in CR1 in the absence of an available matched sibling donor. They also reached the same conclusion when comparing ASCT to allo-transplants with matched unrelated donors [82] in patients who rapidly reached CR1 (so called rapid remitters).
For good-risk patients, the clinical value of allo-transplantation and ASCT has long remained unsettled. Nonetheless, among 2983 patients analyzed from seven published randomized protocols, Schlenk et al. [83] retrospectively identified 124 patients who had AML with double mutant CEBPA and achieved CR1. Relapse-free survival was significantly superior in patients receiving an allo-transplant or an ASCT in CR1 as compared with chemotherapy, whereas OS was not different. Interestingly these data from randomized studies matched the results of an earlier retrospective study from the EBMT [84] which showed similar outcomes post allo or auto in good-risk patients carrying inversion 16 or t(8;21) transplanted in CR1. Since the EBMT registry has no data on patients treated with chemotherapy only, no such comparison was feasible.
The question of the best consolidation regimen for patients after reaching CR1 has been studied by numerous teams in intermediate-risk patients. The Hovon group [85] using the European Leukemia Net (ELN) 2010 prognostic classification, has reported similar OS in this patient category, following a reduced intensity conditioning allo-transplant or an ASCT.
Several teams have reported superior results in patients consolidated while in CR and no detectable MRD. As an example, Messina et al. [86] evaluated WT1 transcript levels in autologous PBSCs from LK used for ASCT in 30 consecutive AML patients in CR and established a correlation with clinical outcome. Real-time quantitative PCR of WT1 was performed in samples of each LK. They defined a cut-off level of 80 WT1-LK copies/ABL 104 copies to discriminate between positive and negative PBSC grafts. This cut-off level was strongly associated with disease recurrence, LFS and OS. Using the EBMT registry, we compared the outcome of 373 patients autografted and 335 patients allografted with a 10/10 compatible unrelated donor in first molecular remission [87]. Patients were stratified using the ELN 2010 classification. We found that good-risk patients benefitted more from autologous transplantation; intermediate-II-risk patients had the same outcome and intermediate-I-risk patients (FLT3-ITD+) benefitted more from unrelated donor transplants. Indeed, the proof of concept sustaining the validity of ASCT for MRD negative patients in CR has been clearly established in acute promyelocytic leukemia, where ASCT has been demonstrated to be the best therapeutic consolidation option in patients who relapsed after first-line therapy and reached a CR2 MRD negative status [88–91]. In the most recent EBMT retrospective study on a large series of adult patients with APL in CR2 receiving allo-transplants (n = 228) or ASCT (n = 341) from January 2004 to December 2018, the 2-year cumulative incidence of NRM was significantly higher for allo-transplants (17.3%) compared with ASCT (2.7%) (p = 0.001), while differences in RI were not significant (28% versus 22.9%; p = 0.28). LFS and OS favored ASCT with 74.5% and 82.4% compared with allo-transplant with 54.7% (p = 0.001) and 64.3% respectively (p = 0.001 and 0.001).
The question of whether intermediate-risk patients reaching CR1 with no detectable residual disease can be consolidated by ASCT and obtain similar outcomes to allo-transplant (with potentially a better QoL) has been addressed by the randomized GIMEMA AML1310 trial of risk-adapted, MRD-directed therapy for young adults with newly diagnosed AML [92]. In this trial of 500 patients, post-remission therapy of young patients was decided by combining the disease risk classification and the post-consolidation levels of MRD. After induction and consolidation, favorable-risk patients (FR) were to receive ASCT and poor-risk patients (PR) allo-transplant. Intermediate-risk patients (IR) were to receive ASCT or allo-transplant depending on the post consolidation levels of MRD. Two-year OS and LFS of the whole series were 56% and 54%, respectively. Two-year OS and LFS were 74% and 61% in the autografted FR risk category and 79% and 61% in the IR MRD-negative category. The investigators concluded that ASCT should have a role in FR and IR MRD-negative categories.
4.4. Maintenance Therapy Post ASCT
Relapse post-transplant remains a major issue in AML. Historically, following ASCT in CR1, the RI has been reported to be from 25% with marrow purged in vitro with mafosfamide, and up to 45% more recently with PBSC at 2 years. Even more concerning is that late relapses occurs with an incidence of 7% at 5 years and 16% at 10 years [93]. This RI may be higher than the 15% usually observed post-allo-transplant.
Historically, stem cell transplantation (allo or auto) has always been considered as the last step in the treatment strategy since additional conventional chemotherapy post-transplant had not demonstrated efficacy. In the last decade however, the development of new therapeutic tools including targeted therapies has renewed interest in this approach. Some retrospective studies have shown a reduction in the RI post allo-transplant with maintenance therapies such as hypomethylating agents (azacytidine IV or p.o.; decitabine) and FLT3-ITD kinase inhibitors [94,95]. Two randomized studies have shown the benefit of sorafenib post-transplant: the German randomized trial (SORMAIN) [96] randomized 83 adult patients with FLT3-ITD-positive AML in complete hematologic remission after HCT to receive either sorafenib (n = 43) or placebo (n = 40). The 24-month LFS probability was 53% with placebo versus 85% with sorafenib (p = 0.002). An open-label, multicenter phase 3 trial in China [97], randomized 202 patients with the FLT3-ITD mutation to receive or not, sorafenib maintenance (400 mg orally twice daily) at 30–60 days post-transplantation. The 1-year cumulative RI was lower (7%) in the sorafenib group than in the control group (24.5%) (p = 0.0010). Similar approaches have not been tested so far after ASCT in good- and intermediate-risk patients but one can postulate a similar reduction in the RI. Furthermore, combinations of a hypomethylating agent (IV or oral) with venetoclax [98,99], or with the anti-47 monoclonal antibody (magrolimab) [100] look even more effective and are currently being tested post-transplant.
4.5. Current ASCT Activity for AML
Data on about 50,000 autologous transplants for AML have been collected since 1980 by the two major registries, the EBMT in Europe and the Center for International Blood and Marrow Transplant Research in North America. But the number of ASCTs for AML in CR1 or CR2 has declined steadily since 2010 and 2015, the EBMT registry has collected annually not more than 300 ASCTs for myeloid malignancies (294 out of a total of 24,418 ASCT carried out in 2018).
Table 1 summarizes the best indications for ASCT in AML, in relation to the disease, the response to induction and consolidation therapy and the MRD evaluation. Some centers still propose ASCT to patients unwilling to receive an allo-transplant and/or to those ineligible according to the recommendations shown in Table 1.
| Patient | AML | Cytogenetics (MRC) | ELNa | Status | CR | Consolidation | MRD | Pre-transplant regimen | Maintenance |
|---|---|---|---|---|---|---|---|---|---|
| Up to 75 years and fit | All but M3 | Good or Intermediate | Favourable or Intermediate | CR1 | Rapid remitterb | At least 2 HD ARA-C courses prior to LK | Negativec | BUMEL or BUCY or CY FTBI | AZA + Venetoclax |
| All three | |||||||||
| FLT3 unmutated | Marrow PB | AZA | |||||||
| LK | CAR-T cells | ||||||||
| M3 | CR2 | Negative | Same |
ELN: European Leukemia Network prognostic classification 2010.
Rapid remitter: reaching complete remission with only one induction chemotherapy course.
No detectable residual disease by either flow cytometry and/or molecular biology.
MRD, Minimal residual disease; MRC, UK Medical research council; HD ARA-C, High dose Cytosine-arabinoside; AZA, 5 Azacytidine.
Guidelines for selecting those patients who may get optimal benefit from autologous stem cell transplantation
The loss of interest as well as this reduced activity has unfortunately precluded the launch of randomized studies that would either compare ASCT to modern chemotherapy, targeted and/or immune therapies, or randomized studies that would evaluate the role of immune therapies post ASCT.
5. AUTOLOGOUS STEM CELL TRANSPLANTATION AS A PLATFORM FOR IMMUNE THERAPIES
The major impediment to a larger use of ASCT as consolidation for AML is in fact the absence of the GVL effect as seen with allo-transplant, despite early attempts at generating some immune anti-leukemic response post-transplant, either with interleukin-2, interferon, or histamine.
Immunotherapy post ASCT has been shown to be of major efficacy for lymphoid malignancies, in particular, with maintenance therapy with anti-CD20 monoclonal antibodies (rituximab or obinutuzumab) in patients with follicular and mantle cell lymphomas [101]. Likewise, the efficacy of lenalidomide post ASCT in multiple myeloma is demonstrated [102,103] and the use of daratumumab maintenance post ASCT is being evaluated [104].
An even more important milestone has been the recent emergence of CAR-T cells, now widely used for the treatment of lymphomas (anti-CD20, CD22) and myelomas (anti-BCMA) and tested in combination with anti PD1 or PD1-L, before, after, or possibly before and after ASCT (https://clinical-hematology.org/special-webinars).
In AML, except for gemtuzumab-ozogamycin which targets CD33, no other effective immune therapy has demonstrated efficacy. Present efforts focus on CAR-T cells with about 22 phase 1/2 trials, some directed against CD33, CD123, FLT3, CD44v6 and the interleukin-1 receptor accessory protein (clinical.gouv NCT03267316) some CAR-T cells being in addition, on/off switchable [105–107]. If and when effective anti AML CAR-T cells become available, the question will arise as to how we will evaluate this new form of highly specific autoimmune lymphocyte infusion that mimics the allo donor lymphocyte infusion and that will bring to ASCT its missing immune anti-leukemic tool (mimicking the allo GVL effect).
6. CONCLUSION
In the past 45 years a considerable number of developments in the field of SCT have occurred and practices have changed. ASCT has become a routine part of the therapeutic platform for lymphoid malignancies and allo-SCT, the best curative treatment for most myeloid malignancies. Several historical transplant indications have almost disappeared, such as chronic myelocytic leukemia. Donors for allogeneic BMT have changed with a reduction in the use of cord blood. Unrelated donors have been replaced by haploidentical donors. ASCT which was very common for AML has remained a therapeutic option for a limited number of teams. It has however remained the best choice when compared with allo-SCT because of a lower NRM and a better QoL. Careful choice of patients based on prognostic score and undetectable MRD will continue to bring outcome benefit, but the future of ASCT will essentially rely on additional tools aimed at reducing the RI.
CONFLICTS OF INTEREST
The author declares no conflicts of interest.
REFERENCES
Cite this article
TY - JOUR AU - Norbert Claude Gorin PY - 2021 DA - 2021/07/15 TI - History and Development of Autologous Stem Cell Transplantation for Acute Myeloid Leukemia JO - Clinical Hematology International SP - 83 EP - 95 VL - 3 IS - 3 SN - 2590-0048 UR - https://doi.org/10.2991/chi.k.210703.002 DO - 10.2991/chi.k.210703.002 ID - Gorin2021 ER -
