Maintenance Strategies Post-Autologous Stem Cell Transplantation for Newly Diagnosed Multiple Myeloma

- DOI
- 10.2991/chi.d.200502.001How to use a DOI?
- Keywords
- Myeloma; Maintenance therapy; Post-ASCT
- Abstract
Multiple myeloma, the second most common hematological malignancy worldwide, has demonstrated dramatic improvements in outcome in the last decade. In newly diagnosed patients, induction chemotherapy followed by autologous stem cell transplantation (ASCT) is the standard of care. After ASCT, the majority of patients experience disease remission but, despite recent therapeutic developments, most will eventually relapse. In this review we consider clinical aspects of maintenance therapies that can be used post-ASCT to prolong remission duration. We discuss the evidence for the effectiveness of each of these drugs as a maintenance therapy, alongside other benefits and drawbacks to their use, for example, route of administration and potential toxicities. We discuss questions which remain unanswered around the optimal use of currently available maintenance therapies and review newer agents being considered for use as maintenance such as emerging immunotherapies.
- Copyright
- © 2020 International Academy for Clinical Hematology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Multiple myeloma (MM) is a malignancy of plasma cells which represents about 2% of all new cancer cases in the UK [1]. In the last decade, there have been major improvements in life expectancy, driven by novel therapeutic agents, autologous stem cell transplantation (ASCT) and improved supportive care [1].
The management of patients with newly diagnosed MM is divided into two broad categories: those fit enough for ASCT and those who would not tolerate such an intensive procedure [2]. Patients eligible for ASCT initially receive induction therapy, usually comprising a combination of three drugs from different classes, for example, a triplet of bortezomib, thalidomide/lenalidomide and dexamethasone (VTD or VRD) [2]. Once the burden of disease has been reduced, an ASCT is undertaken to reinforce the response. Post-ASCT, patients may receive consolidation and/or maintenance medication to prolong remission [3]. Consolidation encompasses the administration of a short course of therapy following ASCT with the aim of deepening response, and maintenance describes the use of long-term medication to prolong remission [4]. Maintenance therapy is typically given for at least 1–2 years post-ASCT and, in many cases, until disease progression [5]. This places a particular importance on the tolerability and ease of delivery for the agents used. Lenalidomide is the only agent currently approved for maintenance therapy post-ASCT both in the US and Europe.
The majority of MM patients respond well to initial induction therapy and disease levels are greatly reduced. Depth of response, measured conventionally by paraprotein levels and, more recently, by minimal residual disease (MRD) assessment, is associated with outcome [6]. However, nearly all patients will eventually relapse [7]. Most can be retreated successfully at relapse, but each remission is associated with diminishing duration and depth of response [7].The first remission is usually the longest and associated with the best quality of life. Therefore the goal of maintenance therapy post-ASCT is to prolong this period, whilst maintaining quality of life, as well as leading to an improved overall survival (OS) [6]. Relapse of MM is caused by residual clonal cells which have survived therapy. Maintenance treatment aims to destroy these remaining malignant clones, either by direct cytotoxicity or by enhancing the immune response against them, when they emerge after a period of quiescence in the bone marrow niche [8].
This review will consider the clinical aspects of the use of maintenance therapy after first ASCT in patients with newly diagnosed MM. To comprehensively review published clinical trials of maintenance agents in this context we searched PubMed using the terms “maintenance” and “myeloma” and “transplant” on 11/06/2019. This search found a total of 752 articles and the abstract of each was reviewed, with relevant studies examined in more depth. Additional information regarding ongoing trials whose outcomes have not yet been published was obtained from US National Library of Medicine resource at www.clinicaltrials.gov.
Clinical trials published to date have predominately explored the use of interferon alpha (IFNα), immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs) (Table 1). We will discuss the potential benefits and drawbacks of each of these agents in the maintenance setting, and also explore unanswered questions about their optimal use [9]. We will then discuss potential future maintenance strategies, the majority of which are immunotherapies.
| Thalidomide | Lenalidomide | Bortezomib | Ixazomib | Daratumumab | |
|---|---|---|---|---|---|
| Mechanism of action | Immunomodulatory agent | Immunomodulatory agent | Proteasome inhibitor | Proteasome inhibitor | CD38 antibody |
| Route of delivery | Oral | Oral | SC (/IV) | Oral | IV (/SC) |
| Frequency | Daily | Daily (21/28 days) | Weekly | Weekly (3/4 weeks) | Monthly |
| Level of evidence | Multiple phase III randomized controlled (including placebo controlled) trials comparing thalidomide to observation demonstrate PFS benefit, OS benefit less clear | Multiple phase III randomzsed controlled (including placebo controlled) trials comparing lenalidomide to observation/placebo demonstrate PFS and OS benefit | Phase III randomized trial comparing VAD induction and thalidomide maintenance to PAD induction and velcade maintenance demonstrated improved in PFS and OS with PAD-velcade | Single phase III randomized, placebo controlled trial demonstrated PFS benefit | Currently under investigation in phase III trials |
| NB no stratification to induction at maintenance randomization | |||||
| Phase III randomized trial comparing thalidomide/bortezomib to thalidomide to interferon demonstrated improved PFS with thalidomide/bortezomib, OS not significantly different | |||||
| Approvals (as at Jan 2020) | – | FDA and EMA approved | – | – | – |
SC, subcutaneous; IV, intravenous; PFS, progression-free survival; OS, overall survival; VAD, vincristine, doxorubicin and dexamethasone; PAD, bortezomib, doxorubicin and dexamethasone; FDA, Food and Drug Administration; EMA, European Medicines Agency.
Summary of maintenance strategies currently in clinical use and those furthest advanced in clinical studies.
2. POST-ASCT MAINTENANCE STRATEGIES
2.1. Early Studies
Maintenance approaches in MM have been under evaluation for over three decades, but have largely been limited by toxicity and delivery problems until the last ten years. Corticosteroids and IFNα were the first agents studied but the use of both was limited by increased toxicity and little evidence of clinically significant benefits.
Corticosteroids are active against myeloma cells and have a wide range of anti-inflammatory and immunosuppressive activities [10]. Prednisolone and dexamethasone have been used as maintenance agents in MM, but were largely studied in the era before ASCT and, therefore, are beyond the scope of this review [11,12].
Interferons are soluble proteins which are naturally produced by cells in response to viruses [13,14]. They were first used as therapeutic agents in MM in the 1970s and have a broad range of anti-proliferative and immune regulatory effects [13,14]. Recombinant IFNα has been used as an induction agent, a therapy at relapse and also in the maintenance setting [13–15]. Several small studies on the use of interferon maintenance with or without corticosteroids were undertaken in patients after ASCT with mixed results [16–18]. Large meta-analyses included both ASCT patients and those who had not undergone the procedure and, overall, there appeared to be a modest progression-free survival (PFS) and OS advantage of around 6 and 4 months, respectively, but this came at the expense of significant toxicity [19,20]. The latter consisted mainly of flu-like symptoms and malaise [16] and, in one study, a third of patients discontinued treatment after a median of only 4 months [18].
2.2. Immunomodulatory Agents (IMiDs)
Thalidomide was initially used in Europe as a sedative anti-emetic in hyperemesis gravidarum and was withdrawn from the market in 1961 due to an association with congenital birth defects [21]. It was later shown to have anti-angiogenic properties and broad immunomodulatory and anti-inflammatory effects [21]. Thalidomide was first found to be useful in MM treatment in the late 1990s, and analogues such as lenalidomide and pomalidomide have subsequently been developed and introduced into clinical practice [21]. The mechanism of action of IMiDs has only recently been elucidated. They bind to the E3 ubiquitin ligase cereblon causing neosubstrates, specifically Ikaros family zinc finger protein 1 (IKZF1) and Ikaros family zinc finger protein 3 (IKZF3), to be targeted for degradation by the proteasome. This, in turn, leads to the downregulation of interferon regulatory factor 4 (IRF4) and MYC, which are critical for myeloma cell survival [22].
2.2.1. Thalidomide
Thalidomide has been thoroughly studied as a maintenance agent post-ASCT with well documented results [6]. Key reports included those of thalidomide alone or thalidomide in combination with prednisolone, compared to observation or placebo. There was a consistent PFS benefit across most studies but the OS difference was variable [23–28]. A meta-analysis of available studies demonstrated a significant late OS benefit [29].
Importantly, the Myeloma IX study was the first to comprehensively characterize adverse cytogenetic lesions by fluorescence in situ hybridization (FISH) in the context of thalidomide maintenance. A subgroup analysis of patients classified as high-risk (defined as the presence of any of the t(4;14), t(14;16), t(14;20), del(17p) or gain(1q)) lesions demonstrated a detrimental association between thalidomide maintenance and high-risk disease for OS [29]. In this study, and several others, thalidomide maintenance was also associated with tolerability issues across the whole population [29]. The median duration of treatment in the Myeloma IX was only 9 months, with half of the patients discontinuing it prior to progression, due to toxicity [29].
As a consequence of common and debilitating side effects, no definitive OS benefit, concern about its use in high-risk patients, and the timely development of second-generation IMiDs, thalidomide maintenance never became a standard of care.
2.2.2. Lenalidomide
Lenalidomide is a second-generation IMiD which is more potent and has fewer side effects than thalidomide [30]. Lenalidomide has been explored as a maintenance agent post-ASCT in several large trials [31]. A meta-analysis evaluated data from 1208 patients (605 in the lenalidomide maintenance group and 603 in the placebo or observation group) [31]. It included data from 3 major trials: Cancer and Leukaemia Group B (CALGB) 100104, Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) RV-MM-PI-209 and Intergroupe Francophone du Myélome (IFM) 2005-02 [32–34]. Of these trials, the first two showed prolonged PFS and OS with the use of lenalidomide, and the last showed prolonged PFS with no significant difference in OS [32–34]. Important differences included the use of a placebo control in the IFM and CALGB studies and the dose of lenalidomide delivered, with a starting dose of 10 mg in the IFM and CALGB studies and 25 mg in the GIMEMA study [32–34]. In addition, patients in the CALGB trial were able to cross over to receive lenalidomide at disease progression [32].
In that meta-analysis, the median PFS was 52.8 months for the lenalidomide group and 23.5 for the placebo or observation group (hazard ratio (HR) 0.48; 95% confidence interval (CI) 0.41 to 0.55) [31]. At a median follow-up time of 79.5 months, the median OS had not been reached for the lenalidomide maintenance group, whereas it was 86.0 months for the placebo or observation group (HR 0.75; 95% CI 0.63 to 0.90; p = 0.001) [31]. The benefit of maintenance was consistent between patients with different depths of response to induction treatment (≤ partial response (PR) and ≥ very good partial response (VGPR), as per the International Myeloma Working Group criteria) [31,35]. Only a small subgroup of patients included in the meta-analysis had molecular risk data available [31]. For those who did, the effect of lenalidomide on PFS was consistent across risk groups; however, for OS, the benefit of lenalidomide over observation/placebo appeared to be significant only in patients with standard risk disease [31].
The UK Myeloma XI trial randomized 730 patients to lenalidomide maintenance and 518 to observation following ASCT, but had not been reported in time to be included in the meta-analysis described above [36]. There was a significant benefit for both PFS and OS associated with the use of lenalidomide maintenance. The median PFS was 57 months for those in the lenalidomide group and 30 in the observation group (p < 0.0001), very similar to the outcomes from the meta-analysis. The 3-year OS was 87·5% (95% Cl 84·3 to 90·7) in the lenalidomide group and 80·2% (76·0 to 84·4) in the observation group (HR 0·69; 95% CI 0·52 to 0·93; p = 0·014) [36]. A meta-analysis incorporating these results with the previous three studies reinforced the original meta-analysis (Figure 1). The Myeloma XI study also included a large number of molecularly characterized patients and demonstrated no significant heterogeneity in terms of the benefit for lenalidomide maintenance compared to observation across all molecular risk groups for both PFS and OS [36].
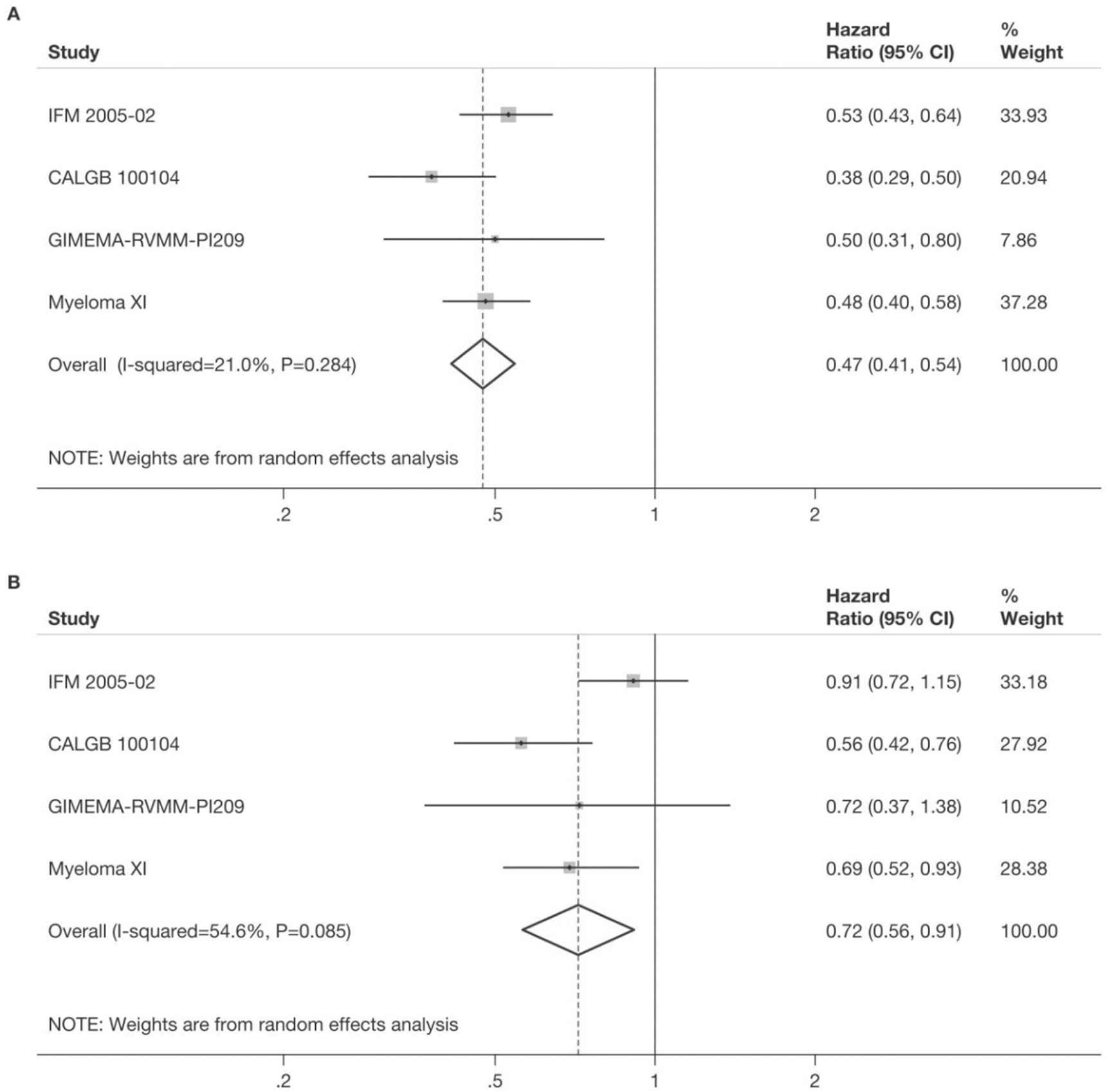
Meta-analysis of studies including a lenalidomide maintenance regimen post-autologous stem cell transplantation (ASCT) for progression-free survival (A) and overall survival (B). Reported estimates of hazard ratios from the relevant studies were combined using the generic inverse-variance method in a random-effects meta-analysis. Hazard ratio <1 indicates a favourable outcome for lenalidomide. Figure previously published in supplementary data of Jackson, GH, et al., Lancet Oncol 2019;20;57–73. [36]
In each of these studies lenalidomide maintenance was better tolerated than thalidomide in the preceding trials, with fewer patients stopping therapy early due to toxicity. However, concerns were raised that there was a slightly increased rate of second primary malignancies in lenalidomide-treated patients. In one study, the incidence of second primary cancers was 3.1 per 100 patient-years in the lenalidomide group versus 1.2 per 100 patient-years in the placebo group (p = 0.002) [33]. This trend was similar across studies, with the cumulative incidence rate of a second primary malignancy higher with lenalidomide maintenance than placebo or observation. However, what is clear is that the cumulative incidence rates of progression, death, or death as a result of MM remain much higher with placebo or observation than with lenalidomide maintenance [31], and the majority of malignancies are non-melanoma skin cancers, suggesting that the benefit of lenalidomide maintenance outweighs the increased risk [37]. Other side effects of lenalidomide maintenance, such as cytopenias and fatigue, are generally well managed with dose reductions. A small percentage of patients suffer diarrhea due to bile acid malabsorption, and this can be managed with bile-salt binding agents to enable patients to remain on therapy [38].
As a result of these studies, lenalidomide is now a well-established standard of care maintenance therapy for MM and is the only approved therapy for this indication.
2.2.3. Pomalidomide
Pomalidomide has greater anti-myeloma efficacy in vitro compared to previous IMiDs [30]. To date there has not been a large randomized control trial assessing pomalidomide maintenance post-ASCT. However, a recent retrospective case series of 7 patients has shown that pomalidomide is well tolerated in this setting, suggesting it may be explored further in future studies [39].
2.3. Proteasome Inhibitors
PIs are active in MM and enhance apoptosis by disrupting the proteasomal degradation of cell cycle and regulatory proteins [40]. The first in class PI, bortezomib, was approved for use in the treatment of MM by the US Food and Drug Administration (FDA) in 2003 and the European Medicines Agency (EMA) in 2004, and has been followed by newer agents such as carfilzomib and ixazomib [40].
2.3.1. Bortezomib
The Spanish Myeloma Group conducted a phase-III trial where patients were randomized to three different induction regimens: VTD (bortezomib, thalidomide and dexamethasone), TD (thalidomide and dexamethasone) and VBMCP/VBAD/B (vincristine, BCNU, melphalan, cyclophosphamide, prednisone/vincristine, BCNU, doxorubicin, dexamethasone/bortezomib) [41]. All groups then underwent ASCT with melphalan conditioning and, after 3 months, patients were randomized to receive maintenance with thalidomide and bortezomib (TV), thalidomide (T) or IFNα. A total of 271 patients were randomized (TV 91; T 88; IFNα 92) [42]. The complete response rate with maintenance was improved by 21% with TV, 11% with T and 17% with IFNα (p not significant) [42]. After a median follow-up of 58.6 months, the PFS was significantly longer with TV compared with T and IFNα (50.6 versus 40.3 versus 32.5 months respectively, p = 0.03) [42]. The OS was not significantly different between the three arms [42].
The HOVON-65/GMMG-HD4 trial also assessed bortezomib as a maintenance agent [43]. In that study, 827 patients were randomized to different induction regimens, namely VAD (vincristine, doxorubicin and dexamethasone) or PAD (bortezomib, doxorubicin and dexamethasone). The two groups underwent ASCT with melphalan conditioning, after which, patients who had been induced with VAD received maintenance with thalidomide, while patients in the PAD group received maintenance with bortezomib [43]. Thalidomide maintenance was delivered as 50-mg capsules daily for 2 years, starting 4 weeks post-ASCT [43]. Bortezomib maintenance was delivered IV at 1.3 mg/m2 once every two weeks for 2 years, starting 4 weeks post ASCT [43]. After a median follow-up of 41 months, the PFS was superior in the PAD+bortezomib maintenance arm (28 months versus 35 months; HR 0.75; 95% CI 0.62 to 0.90; p = 0.002) [43]. In multivariate analysis, the OS was also improved in the PAD+bortezomib maintenance arm (HR 0.77; 95% CI 0.60 to 1.00; p = 0.049) [43]. There was a slightly higher rate of infections and thrombocytopenia with bortezomib maintenance and peripheral neuropathy with thalidomide maintenance [43]. The improvement in PFS and OS associated with the bortezomib-containing regimens persisted across subgroups with high-risk genetics, particularly those with del(17p) [43]. However, it is difficult to evaluate the benefit specific to bortezomib as a maintenance regimen in that study, as the patients in both arms received different induction regimens and, so, the final outcome could be related to the combined effect of both induction and maintenance.
Although it is important to note that bortezomib carries a risk of peripheral neuropathy, this is somewhat attenuated when the drug is administered subcutaneously, and delivery via this mode became more common since these trials were undertaken [44].
2.3.2. Ixazomib
More recently, the second-generation PI ixazomib was developed. Ixazomib has a similar mechanism of action to bortezomib, but is more potent in vitro, and is administered as an oral tablet once weekly [45].
The role of ixazomib as a maintenance agent has been explored in a large (n = 656), double-blind, placebo-controlled trial [46]. In that study, patients were randomized to receive ixazomib or placebo once weekly for 3 weeks out of a 4-week cycle [46]. Patients commenced on a dose of 3 mg ixazomib, which increased to 4 mg if tolerated from cycle 5. Treatment was planned to continue for 24 months or until disease progression or unacceptable toxicity, if earlier. With a median follow-up of 31 months, the PFS was 26·5 months in the ixazomib group versus 21·3 months in the control group (HR 0·72; 95% CI 0·58–0·89; p = 0·0023) [46]. The OS has not yet been published, and data collection is ongoing [46]. The PFS benefit for ixazomib over placebo was consistent across subgroups of patients with a ≤PR or ≥VGPR response, with high- or standard-risk cytogenetics and with measurable or no measurable residual disease (MRD) [46]. There was no difference in second primary malignancies between those treated with ixazomib or placebo [46]. There was no difference in health-related quality of life between patients taking ixazomib and placebo [47].
2.3.3. Carfilzomib
Carfilzomib is a more potent PI than both bortezomib and ixazomib in vitro, and has a slightly different mechanism of action, having been found to bind irreversibly to the proteasome [6]. No studies have been published to date examining the use of carfilzomib as post-ASCT maintenance, but it is being explored in the phase-2 FORTE study (NCT02203643), which includes a maintenance randomization between carfilzomib plus lenalidomide and lenalidomide alone. There is also a phase-3 trial in progress comparing carfilzomib, lenalidomide and dexamethasone versus lenalidomide alone as a maintenance therapy post-ASCT (NCT02659293) [48].
2.4. Immunotherapy Approaches
2.4.1. Monoclonal antibodies
Daratumumab, which targets cluster of differentiation 38 (CD38), and elotuzumab, which targets signaling lymphocytic activation molecule family member 7 (SLAMF7), are both FDA and EMA approved for MM therapy, and there are a number of other monoclonal antibodies (mAbs) being explored [49]. The use of mAbs as maintenance therapeutics post-ASCT is currently being studied, most frequently the use of daratumumab as a single agent or in combination [50]. Daratumumab maintenance post-ASCT is being compared to observation in the phase-3 Cassiopeia trial (NCT02541383), and daratumumab in combination with lenalidomide compared to lenalidomide alone is being studied in the phase-2I GRIFFIN trial (NCT02874742) and in the phase-3 AURIGA trial (NCT03901963). The addition of daratumumab to ixazomib maintenance is being studied in the phase-2 EMN18 trial (NCT03896737). The combination of elotuzumab and lenalidomide maintenance is also being compared to lenalidomide alone in the phase-3 GMMG-HD6 trial (NCT02495922) [48].
3. POTENTIAL POST-ASCT MAINTENANCE STRATEGIES FOR THE FUTURE
Several new immunotherapeutic approaches are in clinical trials for myeloma at more advanced stages of disease, but may represent potential maintenance options in the future.
3.1. Bispecific Antibodies
A bispecific antibody is an engineered protein that is designed to recognize two different epitopes [51]. CD3-B cell maturation antigen (BCMA) bispecific antibodies, such as the bispecific T-cell engager AMG420/BI836909, have been studied in relapsed refractory myeloma with encouraging outcomes, not dissimilar to those seen with BCMA-targeting chimeric antigen receptor (CAR)-T cells (see below) [52]. This BiTE antibody links a single chain antibody variable fragment (scFv) that binds to the invariant CD3 part of the T-cell receptor to another scFv which binds to BCMA on the surface of the myeloma cell. In this way, the BiTE brings a T-cell and a myeloma cell together, which causes T-cell activation, proliferation and tumor cell lysis [53]. The use of bispecific antibodies in the maintenance setting would aim to improve immune surveillance against residual myeloma cells. One study currently recruiting examines the use of blinatumumab (CD3-CD19 bispecific) in combination with second ASCT (NCT03173430) [54].
3.2. Adoptive Cellular Therapies
CAR T-cells are being explored for use in many hematological malignancies, including MM. T-cells are genetically engineered to express an artificial CAR which confers the cells with anti-tumor activity [55]. At present, the most validated target for the CAR in MM is BCMA. BCMA is selectively expressed on both normal and malignant plasma cells, and several studies have looked at their use in relapsed/refractory patients [55,56]. Ongoing trials are evaluating the use of CAR-T cells delivered post-ASCT as a form of maintenance therapy, particularly for those with high-risk disease. CAR-T cells delivered post-ASCT are therefore delivered once the majority of disease bulk has been treated, and they can then act to enhance immune surveillance. Of interest in this regard, alongside BCMA targeting cells, are CAR-T cells targeting CD19. Although MM cells do not normally express CD19, there may be a small “tumor-initiating compartment” of cells that are CD19 positive and are responsible for MRD and relapse [57,58]. In addition, there are many other possible CAR targets including CD38, CD138, Kappa light chain and SLAMF7 [55].
However, there are some limitations with using this type of cellular therapy. At present, CAR-T cells are generated from autologous T-cells for each individual patient. This is costly and time consuming [59]. Allogeneic products could overcome these limitations, but may lead to graft versus host disease (GVHD) via their native T-cell receptor [59]. With both approaches there is also a risk of systemic side effects after cell infusion, including cytokine release syndrome.
NK cells are a type of cytotoxic lymphocyte that forms part of the innate immune system [59]. They express germline-encoded activating and inhibitory receptors that recognize ligands on target cells. NK cells are therefore of interest because allogenic NK cells should not cause GVHD and could act as an “off the shelf” cellular immunotherapy. They can be altered by genetic engineering to improve longevity and cytotoxicity, and their cytotoxic activity can be targeted to a specific antigen using a CAR [59].
3.3. Vaccines
An alternative approach to enhancing immune surveillance post ASCT is the use of therapeutic tumor vaccines. There are many forms of these vaccines currently under investigation, including tumor cell, dendritic cell (DC), protein or peptide-based and genetic vaccines [60]. In MM, vaccines have been used post-ASCT in an attempt to maintain response and extend remission times [61], as, for example, in the phase-2 trial of the idiotype-pulsed DC vaccination, APC8020 (Mylovenge) post-ASCT (both first and subsequent transplant) [62]. This vaccine is a cell-based cancer vaccine composed of autologous DCs pulsed with tumor-derived clonal immunoglobulins [63]. When the vaccine is given to a patient, the idiotype (Id) protein structures can be recognized by antibodies and subsets of T-cells, and this may stimulate anti-tumor cytotoxic T-cells and antibody responses against Id-expressing tumor cells [63]. The study had 27 patients in the intervention arm and 124 in the control arm [62]. The median OS for the trial patients was 5.3 years compared to 3.4 years for the control group (p = 0.02), and the median PFS was similar for the two groups [62]. Vaccines have also been proposed in combination with other therapies, for example, an anti-PD-1 antibody alongside a DC/myeloma fusion vaccine post-ASCT (NCT01067287) [61].
4. COMPARING DIFFERENT MAINTENANCE OPTIONS
There is currently a lack of randomized trials directly comparing different maintenance options. To date, attempts to overcome this lack of data have included retrospective analyses of case series and network meta-analyses. One retrospective study compared lenalidomide and bortezomib maintenance, with 92 patients receiving lenalidomide and 64 receiving bortezomib post-ASCT [64]. There was no difference in PFS or OS between the two groups. Nine patients in the lenalidomide group (9.8%) and 8 in the bortezomib group (12.5%) had adverse events severe enough to necessitate early discontinuation of maintenance therapy [64]. However, retrospective analyses are limited by potential bias and confounding factors, such as those that may have influenced which therapy was selected, something which can be avoided in randomized studies.
Network meta-analyses include data from randomized controlled trials (RCTs) to infer comparisons between interventions when they were studied against a common comparator. The largest reported to date included data from seven trials of post-ASCT maintenance, including the maintenance regimens lenalidomide, thalidomide, bortezomib/thalidomide, lenalidomide/prednisolone and INFα [65]. Lenalidomide-based regimens were identified as being associated with the best improvement in PFS, and bortezomib/thalidomide and thalidomide also showed a beneficial HR [65]. For OS, only lenalidomide maintenance was associated with a benefit [65].
Ongoing studies are attempting to address the data-gap in randomized trials comparing maintenance agents. As an example, a phase-2 trial is currently comparing the use of ixazomib versus lenalidomide maintenance post-ASCT and consolidation in patients with newly diagnosed MM (NCT02253316) [66]. Follow-up is ongoing, but the initial results suggest an increased rate of cessation of maintenance due to disease progression in the patients assigned to ixazomib compared to lenalidomide (cessation 30% and 18%, respectively) [66].
As well as comparing the use of single agents to each other, maintenance therapies could also be used in combination or in different sequences. Combination studies underway are described above and a few early phase studies looking at sequencing agents have also been undertaken [67].
5. IMPACT ON HEALTHCARE UTILIZATION AND PATIENT PREFERENCES
Patient preference around the use of maintenance therapy is increasingly being studied. The period following first ASCT is often the time point in a MM patient's disease course with the longest duration of remission and, so, tolerability of maintenance therapy is of key importance. One study found that lenalidomide or other maintenance therapy post-ASCT did not negatively impact patients' health-related quality of life compared to no maintenance therapy [68].
The economic costs of maintenance are also important in all health systems, and the cost-effectiveness of lenalidomide maintenance therapy has been assessed in several countries. A study performed in The Netherlands showed lenalidomide maintenance to be cost effective when compared to the Dutch willingness-to-pay (WTP) threshold [69]. The use of only the Dutch recommended dose (10 mg with dose reductions if needed) yielded an incremental cost-effectiveness ratio (ICER) of EUR 30,709 [69]. A National Institute of Clinical Excellence (NICE) review of lenalidomide maintenance therapy in the UK is currently underway. A study in Spain analyzed data from two clinical trials [32,33,70] and found a higher incremental cost-utility ratio and ICER than the Dutch study, concluding that the results suggested uncertainty about the appropriate duration of therapy [71]. However, potential limitations of this study include the use of a ten-year time assessment, which might not have included all of the relevant outcomes for patients post-ASCT, and the reported total cost of lenalidomide maintenance therapy which was based on a total treatment duration of 65 months, whereas many trials report a shorter median duration of 25–35 months [72].
A pan-European analysis examined the cost of lenalidomide post-ASCT across 5 European countries (France, Germany, Italy, Spain and the UK) [73]. A model was created to compare the direct costs over 5 years post-ASCT, through having maintenance or no maintenance and up to 2 lines of therapy following disease progression [73]. Two possibilities were assessed, one in which lenalidomide maintenance was given to 80% of eligible patients (LEN MT), and one in which only 20% of eligible patients received lenalidomide therapy (no MT) [73]. The team found that the direct medical costs per patients with LEN MT were €209,600 over the 5-year period and €276,900 for no MT patients [73]. In the no MT group the annual cost increased significantly over the 5 year period, as new lines of therapy needed to be used, and in the LEN MT group the costs decreased, largely due to increased PFS [73].
Studies have assessed other important factors such as hospitalization rates. One study looked at hospital utilization of patients receiving lenalidomide maintenance, any maintenance and no maintenance [74]. They found the rates of hospitalization were similar across the three groups, but that the median duration of hospitalization was longer for the group with no maintenance [74]. The rates of bisphosphonates, growth factors and neuropathic pain medications were also similar across the three groups [74].
Overall, lenalidomide maintenance is well tolerated by patients, has minimal impact on their quality of life and leads to no increase in hospitalization. It is also likely to prove highly cost-effective, although this may vary between countries, depending on different systems of reimbursement.
6. DISCUSSION
The available data suggest that maintenance treatment in newly diagnosed MM patients post-ASCT may deepen response and increase PFS and OS. Lenalidomide is the standard of care for maintenance treatment in many European countries and the USA, and is the only therapy licensed by the FDA and EMA for use in this setting. PIs are also in use, although not yet approved for this indication. Several novel agents are under investigation as maintenance options, including immunotherapeutic approaches. Aside from new therapeutics, there remain two critical unanswered questions with regard to the optimal schedule and delivery of maintenance therapy.
6.1. Risk-Adapted Maintenance
Data on the use of maintenance therapy in the subgroup of patients with high-risk disease are limited. To date, no studies have carried out a randomized comparison powered to determine a difference in this population, but rather rely on subgroup analysis of trials recruiting patients of all risk groups. The Myeloma IX trial demonstrated an adverse outcome associated with the use of thalidomide maintenance in patients with high-risk disease [29]. Conversely, lenalidomide maintenance was able to prolong PFS compared to observation in patients with high-risk disease across several studies and, additionally, prolonged OS compared to observation in patients with high-risk disease in the Myeloma XI trial [36]. In these studies, the effect of adverse risk is ameliorated, but not completely abrogated, suggesting additional or alternative therapies are needed to further improve outcomes for high-risk patients.
The HOVON-65/GMMG-HD4 trial demonstrated a PFS and OS benefit for PAD+bortezomib compared to VAD+thalidomide in patients with high-risk disease, particularly those with del(17p), and this has led some investigators to preferentially chose bortezomib maintenance for patients in that disease category [43]. However, this does not take account of data that suggest thalidomide maintenance may be detrimental to outcome compared to observation in high-risk patients, therefore possibly diminishing outcomes in the other arm of the trial. It also does not take account of the fact that patients were not re-randomized post-ASCT and, thus, had very different induction regimens. It is therefore difficult to extrapolate these findings to other induction settings.
Rather than switching to a different agent, it is likely that high-risk patients may need more than just single agent lenalidomide maintenance, and early studies of combination approaches using lenalidomide plus bortezomib have provided data to support this approach [75]. Ongoing randomized trials looking at PIs or mAbs plus lenalidomide, as described above, will inform this debate further. High-risk patients are also a group in which novel cellular therapies are being explored earlier in the disease course, due to a high unmet clinical need.
6.2. Duration of Maintenance Therapy
Initially, all of the trials leading to the FDA approval of lenalidomide planned to deliver maintenance therapy until disease progression. Shorter duration of maintenance therapy would clearly be cheaper, but it has yet to be demonstrated that this will not sacrifice ongoing disease control. The IFM-2009 trial delivered lenalidomide maintenance for only one year and there is a portion of the trial, undertaken in the USA and not yet reported, which planned to deliver lenalidomide maintenance until disease progression [76]. This will allow a comparison of time limited versus continuous approaches.
A new generation of trials is also underway to determine whether the use of MRD monitoring could be used to determine if and when maintenance therapy could be stopped. It is important this is done with a randomized comparator, as making comparisons between different studies of different agents with different durations makes it very difficult to draw concrete conclusions. The optimal cut off for MRD detection to use in this setting is unknown. Data from the Myeloma XI trial suggests that there is a benefit of lenalidomide maintenance over observation in patients with MRD negativity, measured by flow cytometry with an assay sensitivity of 10−4/5, at the start of therapy and at 6 months post-ASCT. However, whether the use of a more sensitive assay for MRD detection (e.g. sensitivity 10−5 or 10−6), or the use of MRD analysis at multiple time points could be used as an indicator to cease therapy has yet to be demonstrated [77]. It seems likely that sustained MRD over a period of time will likely be better able to predict whether patients can stop maintenance therapy than analysis at one time point.
Answering these remaining questions, as well as incorporating new agents into maintenance strategies, will help us to personalize maintenance therapy and further improve outcomes for myeloma patients.
CONFLICT OF INTEREST
S.B. has no relevant conflicts of interest to declare. GHJ has received consultancy fees and honoraria from Amgen, Janssen, Roche and Merck Sharp and Dohme and consultancy fees, honoraria, research funding and travel support from Celgene and Takeda. C.P. has received consultancy fees, honoraria and travel support from Amgen, Celgene, Janssen and Takeda.
AUTHORS' CONTRIBUTIONS
SAB, GHJ and CP designed the study, SAB performed literature search, SAB and CP performed literature review and drafted the manuscript. All authors reviewed and revised the manuscript and approve the final version submitted.
Funding Statement
C.P. is a National Institute for Health Research Clinical Lecturer, S.B. is a National Institute for Health Research Academic Clinical Fellow.
ACKNOWLEDGMENTS
The authors acknowledge that many investigators have contributed to this field over the years and apologize if, due to a lack of space, any colleague's work has not been mentioned.
REFERENCES
Cite this article
TY - JOUR AU - Sarah A. Bird AU - Graham H. Jackson AU - Charlotte Pawlyn PY - 2020 DA - 2020/05/20 TI - Maintenance Strategies Post-Autologous Stem Cell Transplantation for Newly Diagnosed Multiple Myeloma JO - Clinical Hematology International SP - 59 EP - 68 VL - 2 IS - 2 SN - 2590-0048 UR - https://doi.org/10.2991/chi.d.200502.001 DO - 10.2991/chi.d.200502.001 ID - Bird2020 ER -
