Timed Sequential Salvage Chemotherapy for Relapsed or Refractory Acute Myeloid Leukemia
Both authors contributed equally.
- DOI
- 10.2991/chi.d.191128.001How to use a DOI?
- Keywords
- Acute myeloid leukemia; Timed sequential chemotherapy; EMA; Chemotherapy; Refractory AML; Relapsed AML; AML
- Abstract
Therapy for those with relapsed or refractory acute myeloid leukemia is suboptimal. Studies have suggested that timed sequential salvage combination cytotoxic chemotherapy may be particularly useful for that indication. We report here a series of ten such adult patients treated sequentially at a single center with EMA (cytarabine 500 mg/m2/day as continuous infusion on days 1–3 and days 8–10, mitoxantrone 12 mg/m2/day on days 1–3, and etoposide 200 mg/m2/day as continuous infusion on days 8–10). The overall complete remission rate was 40% (including 3 of 4 of those with relapsed disease), but use of this regimen was associated with prolonged cytopenia and a high rate of infectious adverse events. Even with the availability of modern infectious prophylaxis and therapies, the EMA regimen is likely best reserved for those with relapsed disease treated with curative intent prior to an allogeneic hematopoietic cell transplant.
- Copyright
- © 2019 International Academy for Clinical Hematology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Novel therapeutic interventions for acute myeloid leukemia (AML) include molecularly targeted agents and immunotherapy, but the traditional backbone of induction therapy remains an anthracycline and cytarabine based regimen [1–4]. Up to 80% of newly diagnosed patients will respond to cytotoxic chemotherapy if eligible to receive it; however, those who do not respond and those who later experience relapse require salvage interventions [5,6]. Genetic profiling of AML prior to treatment allows stratification into subgroups with differing prognosis [1], yet failure to achieve or maintain a complete remission (CR) confers a dismal outcome irrespective of initial risk group [6–9]. While there are many options for relapsed/refractory (R/R) AML patients, there is no consensus on the optimal approach other than the enrollment in clinical trials whenever available [9].
Aside from patients with known actionable mutations, the predominant regimens used for salvage therapy remain chemotherapy-based [9]. Timed sequential therapy (TST) is an approach aimed to enhance the anti-leukemic effect, by using an initial sequence of chemotherapy followed by a subsequent second sequence of cell-cycle active drugs at the peak time of recruitment of AML cells in the S phase of the cell cycle induced by the first sequence [10–12]. Archimbaud et al. demonstrated the clinical utility of such an approach with the EMA regimen including administration of cytarabine and mitoxantrone on days 1–3, followed by cytarabine and etoposide on days 8–10, with an overall CR rate of 61% [13]. In a randomized double-blind phase III study (EMA91), the addition of granulocyte-macrophage colony-stimulating factor (GM-CSF) aimed at increasing the recruitment of AML cells in the S phase of the cell cycle between the first and second sequence of chemotherapy; however, no difference was seen in CR rates between the two arms [14]. Over thirty years have passed since the development of EMA, in which advancements have occurred in blood banking services, infectious disease prophylaxis and treatment, and bone marrow transplant. Therefore, we wanted to re-assess the use of TST in the modern era.
Herein, we report a series of ten R/R AML patients treated at a single institution with the EMA86 regimen.
2. METHODS
All patients provided written informed consent and were treated on National Heart, Lung, and Blood Institute (NHLBI) IRB-approved protocol (NCT00001397) with co-enrollment on laboratory protocol PEARL15 (NCT02527447) [15] at the of the National Institutes of Health (NIH) Clinical Center. Patients with non-APL AML, no prior hematopoietic cell transplant and ECOG performance status ≤ 2 were included. Baseline clinical characteristics are presented in Table 1. We considered as relapsed AML the re-occurrence of leukemia in patients who obtained at least one CR, whereas the patients who failed to achieve CR after one or more cycles of induction were referred to as refractory.
| Median age, years (range) | 56 (23–66) |
| Gender n (%) | |
| Male | 4 (40) |
| Female | 6 (60) |
| Relapsed/refractory status, n (%) | |
| Relapsed | 4 (40) |
| Refractory | 6 (60) |
| WHO AML subtype, n (%) | |
| AML with myelodysplasia related changes | 4 (40) |
| AML with t(8;21) | 1 (10) |
| AML NOS with monocytic differentiation | 2 (20) |
| AML NOS | 3 (30) |
| Median bone marrow blast infiltrate at admission, % (range) | 35 (19–58) |
| Median WBC count at admission, n/µL (range) | 2,425 (300–42,230) |
| MRC cytogenetic risk, n (%) | |
| Favorable | 1 (10) |
| Intermediate | 4 (40) |
| Adverse | 4 (40) |
| Normal karyotype | 1 (10) |
| ELN risk score, n (%) | |
| Favorable | 2 (20) |
| Intermediate | 2 (20) |
| Adverse | 6 (60) |
| Previous chemotherapy, n (%) | |
| < /= 2 previous lines | 8 (80) |
| >2 previous lines | 2 (20) |
AML = acute myeloid leukemia; ELN = European Leukemia Network; MRC = Medical Research Council; NOS = not otherwise specified; WBC = white blood cell; WHO = World Health Organization.
Baseline patient clinical characteristics.
All patients underwent pre-treatment bone marrow aspiration and trephine biopsy assessment with morphological and immunohistochemical evaluation, cytogenetics and flow cytometry analysis. Baseline molecular characterization to detect mutations in 54 genes frequently mutated in AML was performed using next generation sequencing (NGS). All patients received chemotherapy according to the EMA-86 protocol (cytarabine 500 mg/m2/day as continuous infusion on days 1–3 and days 8–10, mitoxantrone 12 mg/m2/day on days 1–3, and etoposide 200 mg/m2/day as continuous infusion on days 8–10).
Response was assessed by bone marrow examinations performed between days 14 and 20 of treatment for interim assessments, and between days 28 and 35 for final response assessment. The CR and complete remission with incomplete hematological recovery (CRi) were defined according to the ELN 2017 guidelines [1]. In addition, early assessment of molecular measurable residual disease (MRD) was performed on days 1 and 4 of EMA for nine of the ten patients using an NGS assay as previously described [15].
Patients received antimicrobial prophylaxis based on NCCN guidelines [16], with fluoroquinolones (n = 8), sulfamethoxazole/trimethoprim (n = 1) acyclovir (n = 10), micafungin (n = 3), posaconazole (n = 3) until the resolution of neutropenia or the escalation of antimicrobial therapy for neutropenic fever. Severity of chemotherapy-related toxicities was assessed according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Effects (CTCAE) v4.0.
3. RESULTS
The patients had a median age of 56 years (range 23–66), and all had an ECOG performance status of 0 or 1. The median white blood cell (WBC) count prior to chemotherapy was 2,425/µL (range 300–42,230/µL) with a median of 13.7% circulating blasts (range 6–80%). The median bone marrow AML infiltrate was 35% (range 19–58%). Four patients had AML with myelodysplasia-related changes, one had AML with t(8;21), three had AML NOS and two AML NOS with monocytic differentiation. Only one patient presented with proliferative disease with a WBC count of 42,230/µL, all the others had leukopenia or a normal WBC count. The most frequent cytogenetic abnormalities identified were trisomy 8 (n = 4), del(7) (n = 2), del(5) or del(5q) (n = 2), del(3) (n = 2), del(9) (n = 2). Three patients had complex karyotype and only one had normal karyotype. The most common molecular variants identified by NGS were mutations in DNMT3A (n = 6), ASXL1, IDH1, IDH2 and TP53 (n = 2) Figure 1. According to the 2017 European Leukemia Network (ELN) risk classification [1], 60% (n = 6) of the patients were classified as adverse, 20% (n = 2) intermediate and 20% (n = 2) favorable risk. All the refractory patients had adverse-risk AML. All patients received at least one cycle of standard induction chemotherapy, with a median of 2.5 cycles of previous total chemotherapy (range 1–6) with 60% (n = 6) of the patients refractory to prior chemotherapy (Table 1).
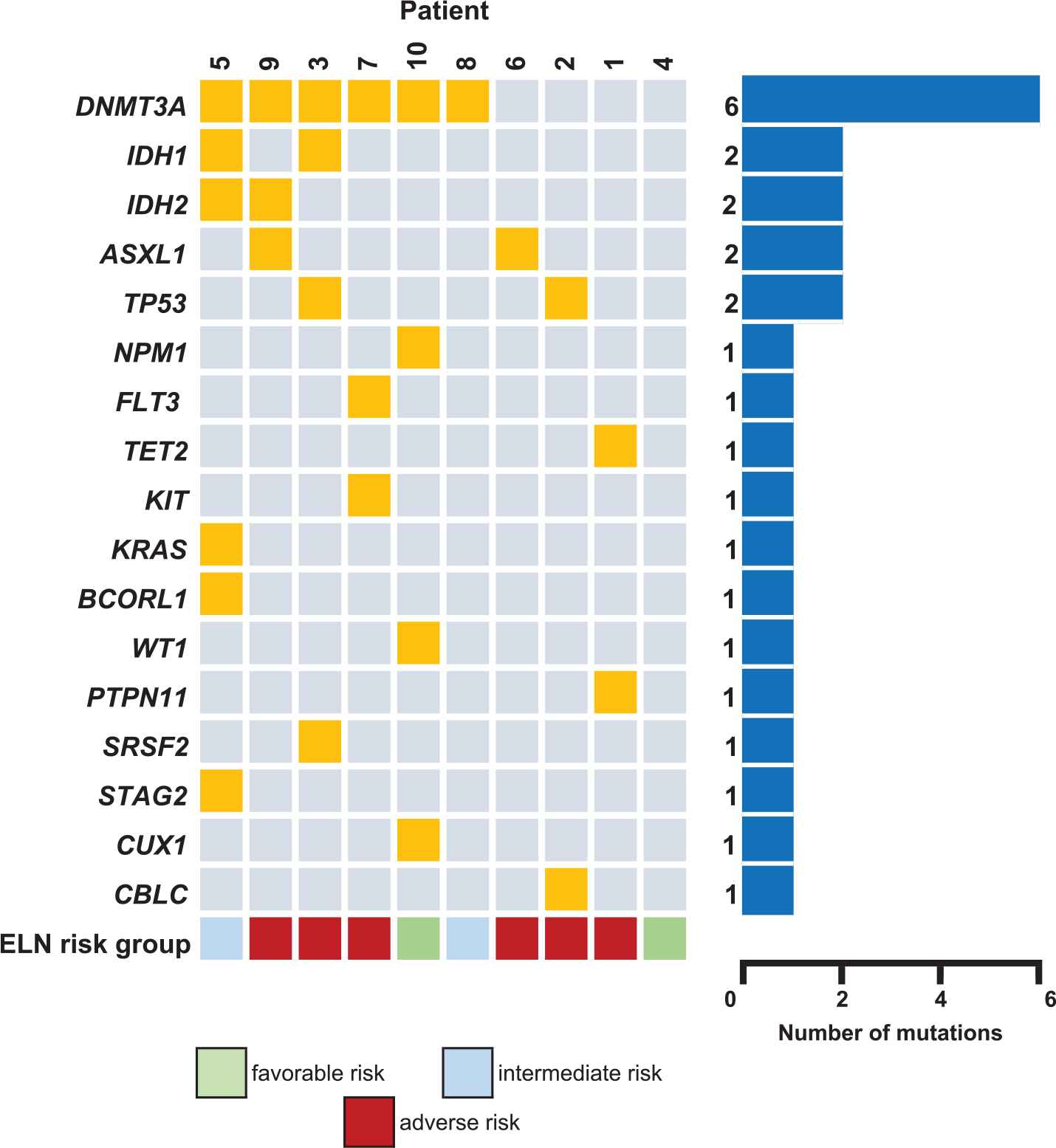
Baseline patient molecular characteristics prior to etoposide, mitoxantrone and cytarabine (EMA) salvage chemotherapy.
The overall CR rate after EMA-86 was 40%, with 75% of the patients with relapsed AML and 17% with refractory disease obtaining CR after salvage chemotherapy (Table 2). Within the adverse ELN adverse risk group, the CR rate was only 16.66% (n = 1), 50% for the favorable and 100% (n = 2) for the intermediate risk group. The median overall survival (OS) was 80.5 days (range 30–1205), being 41 (range 30–116) days in refractory and 332.5 days (range 42–1205) in relapsed patients. Two patients were refractory to EMA-86 and did not receive any further treatment, and two were refractory to EMA-86, enrolled in other protocols and eventually died from disease progression. Two patients underwent allogeneic hematopoietic cell transplantation (alloHCT) in CR: one died due to transplant-related veno-occlusive disease (VOD) and the other remains in persistent CR at more than 1,000 days post-transplant [17]. Another two patients obtained CR from EMA-86, received consolidation regimens outside the NHLBI and were leukemia-free at the last follow-up.
| Clinical Responses |
||
|---|---|---|
| Relapsed (n = 4) | Refractory (n = 6) | |
| Response, n (%) | ||
| CR | 2 (50) | 1 (17) |
| CRi | 1 (25) | 0 (0) |
| RD | 1 (25) | 5 (83) |
| Hematological recovery, n (%) | ||
| ANC > 1,000/µL | 4 (100) | 1 (17) |
| Platelets > 100,000/µL | 2 (50) | 2 (33) |
| Overall survival, days, median (range) | 332.5 (42–1205) | 41 (33–116) |
| Cause of death, n (%) | ||
| Sepsis | 0 (0) | 2 (33) |
| AML progression | 1 (25) | 3 (50) |
| AlloHCT complications | 1 (25) | 0 (0) |
| Length of hospitalization, days, median (range) | 42.5 (38–74) | 41 (33–60) |
AML = acute myeloid leukemia; ANC = absolute neutrophil count; CR = complete remission; CRi = complete remission with incomplete hematological recovery; RD = refractory disease; HCT = hematopoietic cell transplant; EMA = etoposide, mitoxantrone and cytarabine.
Clinical outcomes following salvage EMA chemotherapy.
The early assessment of MRD identified NGS-trackable variants in 70% of the patients (n = 7). All variants present in the peripheral blood at day 1 of EMA remained detectable at day 4, with consistent variant allele frequencies, and were not contributors to the clinical response prediction [15].
Among the ten patients, only half managed to recover the absolute neutrophil count (ANC) to more than 1,000/µL and only four recovered the platelet count to 100,000/µL (Figure 2). The median recovery time was 38 days (range 32–57) and 43 days (range 33–46) for ANC and platelets, respectively, with a median duration of hospitalization of 42.5 days. All relapsed patients recovered their ANC. Despite receiving antimicrobial prophylaxis, all patients developed febrile neutropenia and six of them had documented localized or systemic infections—fungal (n = 4), bacterial (n = 2) or mycobacterial (n = 1)—requiring broad spectrum antibiotic and prolonged antifungal treatment courses. Two patients developed invasive aspergillosis, one patient had disseminated tuberculosis, and one patient had systemic infection with fusarium (pulmonary, sinusal and cutaneous) [17], with two patients eventually dying of sepsis and multiple system organ failure. The toxicities associated with the administration of EMA are summarized in Table 3.
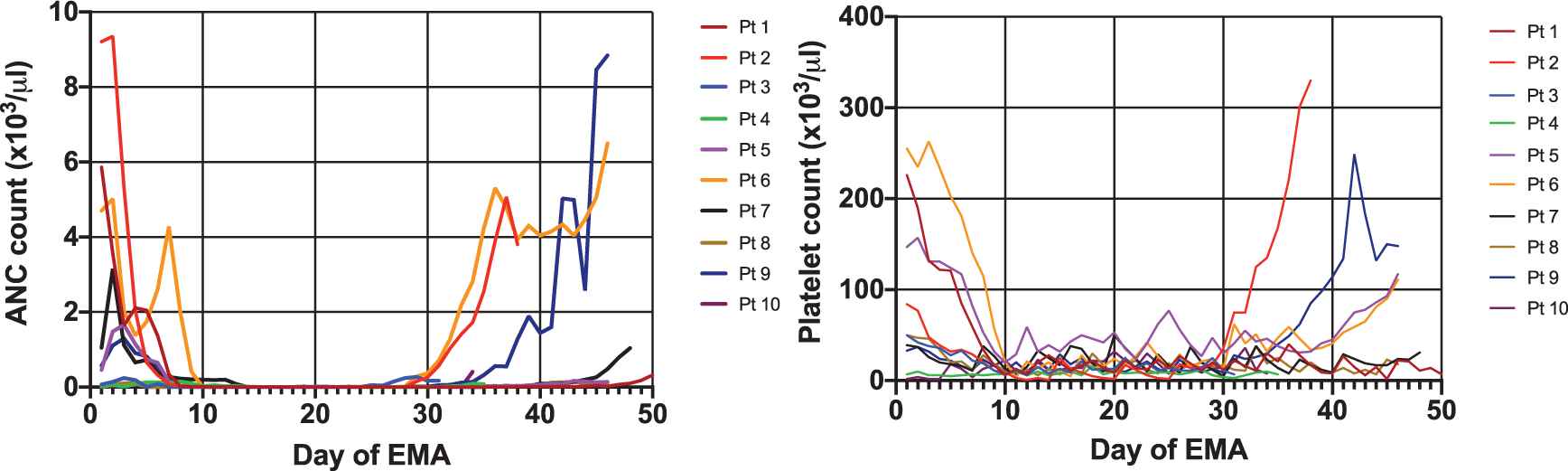
Absolute neutrophil count (ANC) and platelet count recovery after etoposide, mitoxantrone and cytarabine (EMA) salvage chemotherapy.
| Adverse Effects According to CTCAE v4.0 |
||||
|---|---|---|---|---|
| Adverse Effect | Grade 3, n | Grade 4, n | Grade 5, n | Total, n |
| Febrile neutropenia | 10 | 10 | ||
| Infectious | ||||
| Catheter related infection | 1 | 1 | ||
| Infectious colitis | 1 | 1 | ||
| Lung infection | 5 | 1 | 6 | |
| Skin infection | 2 | 2 | ||
| Sinusitis | 1 | 1 | ||
| Sepsis | 3 | 2 | 5 | |
| Gastro-intestinal | ||||
| Mucositis | 5 | 5 | ||
| Typhilitis | 1 | 1 | ||
| Appendicitis | 1 | 1 | ||
| Pancreatitis | 1 | 1 | ||
| Renal | ||||
| Acute kidney injury | 1 | 1 | ||
| Metabolic | ||||
| Hypernatremia | 1 | 1 | ||
| Skin | ||||
| Rash | 2 | 2 | ||
CTCAE = common terminology criteria for adverse effects; EMA = etoposide, mitoxantrone and cytarabine.
One patient died with concurrent lung infection and sepsis.
Toxicities during EMA salvage chemotherapy.
4. DISCUSSION
R/R AML patients have an overall poor prognosis, with a 5-year OS of 5–10% [18]. There is no consensus on the superiority of an individual salvage therapy, and no difference in survival between most non-targeted regimens. A multicenter randomized phase III clinical trial to investigate elacytarabine versus investigator's choice in 381 R/R AML did not show any significant difference in OS among any of the eight regimens investigated, with an overall CR rate of 22%, emphasizing the lack of an effective standard of care for R/R AML patients [19].
TST with cytarabine, mitoxantrone and etoposide as salvage chemotherapy previously showed encouraging efficacy with a CR rate of 61% (45% of refractory and 81% of relapsed AML patients) as a single-arm study [13]. Our small single institution experience showed 40% of the patients achieved CR or CRi (17% of refractory and 75% of relapsed patients), comparable to results from other salvage intensive chemotherapy studies [19,20].
A major benefit of achieving a CR after a salvage regimen in R/R AML patients is the opportunity to undergo alloHCT with curative intent. Two of the patients in our cohort were eligible for the procedure and were transplanted in CR after EMA; one of them was alive with no measurable residual AML detectable at the last post-transplant follow-up, and the other one died of transplant-related VOD without any evidence of persistence of leukemia. All the patients treated in this series had a ECOG performance status of 0 to 1, and had a plan to go to alloHCT following achievement of remission. An alternative approach to intensive cytotoxic salvage chemotherapy is to perform alloHCT after timed sequential conditioning to reduce the leukemic burden before engraftment. The combination of initial cytoreductive chemotherapy followed by reduced-intensity conditioning (RIC) and donor lymphocyte infusion (DLI) showed a 2-year OS of 46% in 103 refractory AML patients [21]. Furthermore, a more recent thiotepa-based RIC sequential approach showed similar efficacy in haploidentical transplants with improved relapse-free survival compared to unrelated donor transplants [22].
The decision to perform or not perform alloHCT, choice of conditioning regimen, and the type of transplant should be based on individual patient factors which weigh risks and benefits of morbidity and mortality. More recently, the MRD status prior to transplant has been recognized as an important prognostic factor in post-transplant outcomes [7]. Assessment of early MRD prior to the standard response assessment time points needs to be further explored. However, very early molecular response measurement by NGS at day 4 of chemotherapy in our analysis did not correlate with the clinical outcome [15].
Interestingly, despite advances in supportive care services over the last 30 years, there were significant toxicities associated with EMA-TST, similar to those reported by Archimbaud et al. in the early 1990s [13]. Prolonged cytopenias and deaths secondary to infection occurred in 20% of the patients, while only 50% recovered the ANC to a value greater than 1,000/µL. Therefore, for this particular regimen, the benefit associated with the scientific approach of prolonged and sequential leukemia cell kill must be carefully weighed against risks associated with prolonged cytopenia.
Overall, our retrospective single institution study shows that even in the modern era, timed sequential chemotherapy has considerable toxicity, and perhaps should be reserved for select relapsed patients where the goal is a potentially curative alloHCT, and for whom novel targeted therapy is not an option.
CONFLICT OF INTEREST
Christopher S. Hourigan has received laboratory research funding from Merck and Sellas. Catherine Lai has served on an advisory board for Agios, Daiichi-Sankyo, and Jazz Pharmaceuticals and as a speaker for Astellas and Jazz Pharmaceuticals. All other authors report not relevant conflicts of interest.
AUTHORS' CONTRIBUTIONS
CSH, CL, SS, JT, SG, TI, and CBS conducted clinical research. BP, JT, and CL did data analysis and interpretation. BP was involved with the first draft of manuscript. BP, CSH, JT, and CL edited the manuscript.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health.
REFERENCES
Cite this article
TY - JOUR AU - Bogdan Popescu AU - Sheenu Sheela AU - Julie Thompson AU - Sophia Grasmeder AU - Therese Intrater AU - Christin B. DeStefano AU - Christopher S. Hourigan AU - Catherine Lai PY - 2019 DA - 2019/12/09 TI - Timed Sequential Salvage Chemotherapy for Relapsed or Refractory Acute Myeloid Leukemia JO - Clinical Hematology International SP - 27 EP - 31 VL - 2 IS - 1 SN - 2590-0048 UR - https://doi.org/10.2991/chi.d.191128.001 DO - 10.2991/chi.d.191128.001 ID - Popescu2019 ER -
