Accurate Measurement of Blood Pressure

- DOI
- 10.2991/artres.k.200624.001How to use a DOI?
- Keywords
- Blood pressure determination; aorta; catheterisation; cuffless
- Abstract
Accurate Blood Pressure (BP) measurement is vital for appropriate diagnosis and management of cardiovascular risk. However, questions remain on the accuracy of cuff BP compared with invasive (intra-arterial) BP. Moreover, the critical physiological factors that are associated with inaccuracy of cuff BP and estimated central BP are still not fully understood. Our group has recently conducted a series of individual participant data meta-analyses, and targeted physiology studies to address these questions and build knowledge on possible ways to improve the accuracy of BP measurements. The aim of this review is to detail this work and briefly discuss future directions for the field.
- Copyright
- © 2020 Association for Research into Arterial Structure and Physiology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Raised Blood Pressure (BP) is the number one modifiable risk factor for cardiovascular disease, which causes approximately one in three deaths globally [1]. Clinical measurement of BP is performed with an inflatable upper-arm cuff and sphygmomanometer. The method was first described by Scipione Riva-Rocci in 1896 [2,3]. In 1905, Nikolai Korotkoff reported five distinct sounds that can be identified by auscultation of the brachial artery during cuff deflation [4]. The most well-known are the 1st and 5th Korotkoff sounds, which correspond to Systolic BP (SBP) and Diastolic BP (DBP) respectively [2]. Since the 1970s automated devices have increased in popularity. The most common method to estimate BP with automated devices is the oscillometric technique. In this method, an oscillometric envelope is generated from oscillations in pressure throughout cuff deflation and device-specific methods are used to estimate BP [5].
Cuff BP is undoubtedly a powerful predictor of cardiovascular risk and is invariably used for management of BP in clinical practice [6,7]. The accurate measurement of BP is fundamental to the optimal detection, treatment and management of raised BP [8]. However, after more than 100 years, questions still remain on the accuracy of cuff BP compared with gold-standard invasive BP measurements. Resolving questions on the accuracy of BP measurement is critically important because as little as 5 mmHg measurement error could result in misclassification of hypertension in 84 million people worldwide [9], and lead to inaccuracies in perceived hypertension prevalence and control rates [10]. The potential implications of underestimated BP are that patients may be left at unnecessarily high cardiovascular risk because lifestyle modification and/or pharmacological treatment has not been initiated. On the other hand, overestimation of BP may lead to initiation of unnecessary lifelong BP medications along with the associated costs and potential side-effects. The aim of this review is to describe our recent work to understand the accuracy of cuff BP compared with invasive BP, critical physiological factors associated with inaccuracy and future directions for the field.
2. ACCURACY OF CUFF BP COMPARED WITH INVASIVE BP
The INvaSive blood PressurE ConsorTium (INSPECT) was established to improve understanding of cuff BP accuracy compared with gold-standard invasive (intra-arterial) BP. Following an extensive systematic review, the consortium compiled data from more than 3000 individual patients from over 30 studies. Recently three distinct, but closely aligned research questions were addressed: (1) invasive aortic BP compared with invasive brachial BP; (2) cuff BP compared with invasive brachial BP; and (3) cuff BP compared with invasive aortic BP. Inclusion criteria for the consortium database included measurement of cuff BP during the invasive BP procedure (not, for example, in a waiting bay prior to the measurement of invasive BP) and measurement of BP under baseline conditions. The full inclusion and exclusion criteria have been published [11].
2.1. Invasive Aortic Compared with Invasive Brachial BP
The original purpose of cuff BP was to measure central aortic BP [3]. This is logical because the BP in the central arteries causes major adverse cardiovascular events, not the BP in the periphery. But, arterial signals from the brachial artery are used for cuff BP, which suggests the measurement should correspond to invasive brachial BP. Therefore, the true level of difference between the aorta and brachial artery (BP amplification) has implications for understanding whether cuff BP measures invasive aortic or brachial BP. In recently published work using the INSPECT database [11], 515 subjects (mean age 59 years, 72% male) from 13 studies, the average invasive aortic-to-brachial SBP amplification was 8.0 mmHg (Figure 1). However, SBP amplification was highly variable among individuals [11], which creates uncertainty on whether cuff BP measures invasive brachial BP or invasive aortic BP. To try to resolve this uncertainty, both these questions were examined using the INSPECT database [11].
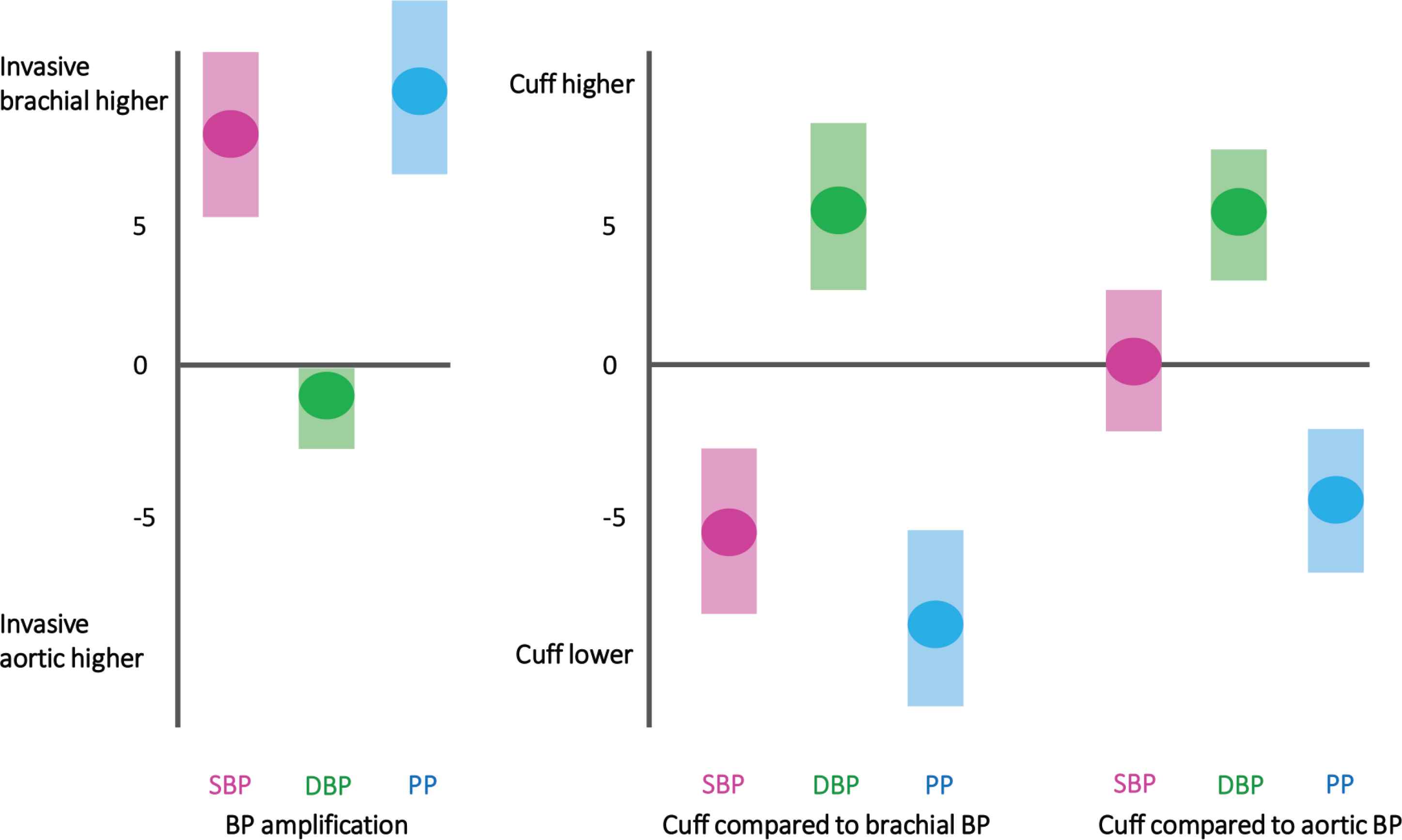
Schematic illustrating level of invasive aortic-to-brachial Blood Pressure (BP) amplification and the differences in cuff BP compared with invasive brachial and invasive aortic BP. (Adapted from Picone et al. [11].)
2.2. Cuff BP Compared with Invasive Brachial BP
In the meta-analysis of cuff and invasive brachial BP, the analysis was conducted on 735 subjects (mean age 53 years, 62% male). Cuff SBP systematically underestimated invasive brachial SBP by −5.7 mmHg, and systematically overestimated invasive brachial DBP by 5.5 mmHg (Figure 1). In a sub-analysis, underestimation of SBP was on average higher using oscillometric compared with mercury sphygmomanometry (−8.0 mmHg vs. −3.4 mmHg), although the mean absolute difference between the two methods was similar (oscillometric: 8.1 mmHg vs. mercury: 7.5 mmHg). These analyses showed that cuff SBP underestimated invasive brachial SBP but because of aortic-to-brachial SBP amplification, cuff BP might be a reasonable approximate of invasive aortic SBP (Figure 1) [11].
2.3. Cuff BP Compared with Invasive Aortic BP
Cuff BP was compared with invasive aortic BP in 1823 subjects (mean age 60 years, 70% male) from 39 individual studies in the INSPECT database [11]. There was no difference between cuff and invasive aortic SBP (0.3 mmHg; Figure 1). However, in many individuals, cuff SBP substantially underestimated or overestimated invasive aortic SBP. This large individual variability was exemplified by only 33% of cuff and invasive aortic (or brachial) SBP measurements falling within ±5 mmHg of each other. Moreover, in the BP range of 120–159/80–99 mmHg (pre-hypertension and stage 1 hypertension according to JNC7 guidelines) [12], there was only 52–57% concordance between cuff and invasive aortic BP [11]. This level of discordance may have major ramifications for accurate classification and management of BP because most of the world’s population has a SBP in the range of 120–159 mmHg [13]. Cuff overestimation of invasive aortic DBP was essentially the same magnitude as the brachial DBP analysis. This was unsurprising because DBP is relatively consistent through the large arteries compared with SBP [11]. A second study recently published from the INSPECT database also showed that invasive aortic SBP and DBP are respectively under- and over-estimated to a greater level with increasing age [14]. Altogether, these data support the need to improve the accuracy of cuff BP. The need for accurate cuff SBP is probably more important than cuff DBP because it is a stronger predictor of cardiovascular risk [15–17].
2.3.1. Technical aspects underlying cuff BP inaccuracy
There are several technical aspects that may contribute to inaccuracy of cuff BP measurements. First, a faster the cuff deflation rate will lead to greater underestimation of SBP and overestimation of DBP [18]. This relationship is also modulated by the patient’s heart rate. Second, for the auscultatory method, there may be a delay between the time the cuff deflation crosses the true SBP and the Korotkoff sound [18]. Third, physiological respiratory variation may also lead to errors in BP measurement [19]. Finally, it is also important to note that there may be some methods that show better agreement with invasive BP than others. For example, wideband recording of the brachial waveform can give excellent agreement with invasive brachial BP, potentially even superior to auscultation [20]. Other issues that may influence the accuracy of cuff BP have previously been discussed in detail [21], and are not the focus of this review.
3. ARTERIAL PHYSIOLOGY UNDERPINNING PROBLEMS WITH CUFF ACCURACY
3.1. Phenotypes of Blood Pressure Amplification and Cuff BP Measurement Accuracy
Amplification of SBP and pulse pressure from the heart to the peripheral arteries is a well-established physiological phenomenon [11,22]. Large individual variability of BP amplification could have implications for the accuracy of cuff BP [11]. In our recently published work [23], we addressed this issue with measurements of invasive BP at the aorta, brachial and radial arteries via sequential catheter pullback, as well as cuff BP. This protocol allowed measurement of SBP amplification from the proximal aorta through the entire upper-limb. The study enrolled 126 patients (mean age 61 years, 69% male) undergoing coronary angiography and four distinct BP phenotypes were discovered based on changes in SBP amplification across the aorta-to-brachial and brachial-to-radial arteries (≥5 mmHg change = SBP amplification; <5 mmHg change = no SBP amplification; Figure 2) [23]. The critical finding was that patients with no aortic-to-brachial SBP amplification (phenotypes three and four) had significantly higher invasive aortic BP compared with those with SBP amplification (phenotypes one and two). Most importantly, there was no difference in standard cuff BP in those with no SBP amplification versus SBP amplification, despite cuff BP being taken with multiple different devices at different times. The observations were confirmed with data from independent investigators in Italy, China and Taiwan.
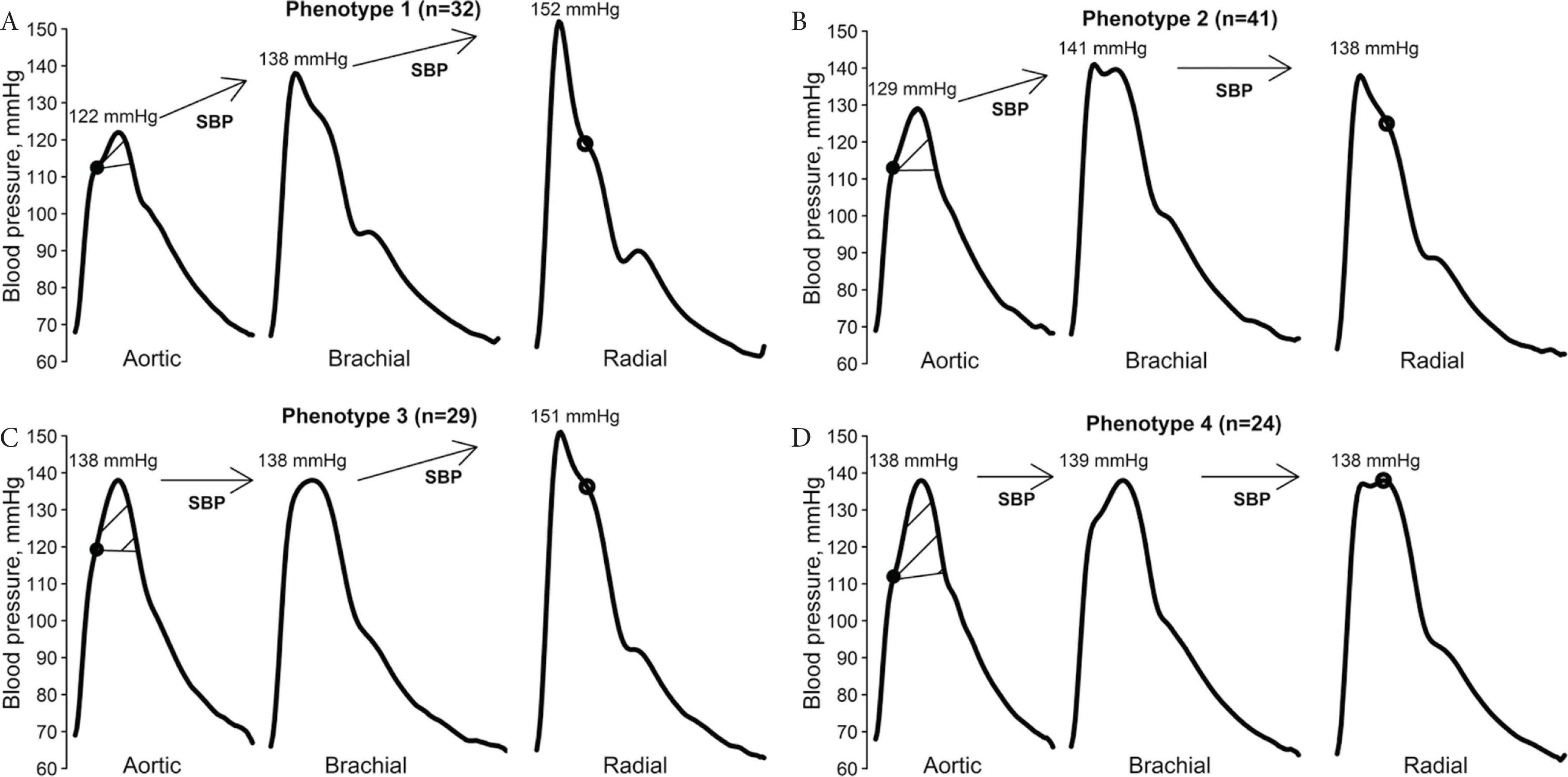
Blood Pressure (BP) phenotypes according to variation in systolic BP amplification from the aorta-to-brachial-to-radial arteries. Figure from Picone et al. [23] and republished in accordance with the AHA Copyright Transfer Agreement.
We hypothesised cardiovascular risk may be higher for those with no aortic-to-brachial SBP amplification versus those with SBP amplification. This was because invasive aortic BP was higher in patients without SBP amplification, despite no difference in cuff BP. However, it is important to note that diagnoses of high BP should be made from multiple BP measurements at different times [2], which was not possible in this study. Our research team are now assessing the clinical relevance of the different SBP amplification phenotypes via associations with target organ damage and a large, prospective trial with follow up to cardiovascular events.
Alongside the potential implications for cardiovascular risk related to BP, the BP physiology related to SBP amplification may provide insights on how to improve the accuracy of cuff BP. Across the different SBP amplification phenotypes there were distinct differences in the arterial waveform shape. Importantly, standard cuff BP methods do not use information from the arterial waveform in the measurement of BP. This neglects a vast amount of physiological information that should be considered in future work to improve cuff BP accuracy [23].
One limitation of this work is that the reproducibility of the SBP amplification phenotypes is unknown because it was not possible to record repeated visit data. Another potential limitation is that the SBP amplification profile may change across a 24-h period and this could influence the phenotype observations. It is not possible to capture 24-h invasive SBP amplification data, but non-invasive ambulatory central aortic BP may provide useful insights on the circadian pattern of SBP amplification.
3.2. Potential Implications of Brachial-to-Radial BP Amplification on Accurate BP Measurement
Most research on BP amplification has focussed on the aortic-to-brachial arteries, not the brachial-to-radial arteries. However, brachial-to-radial SBP amplification could be important for accurate measurement of BP from ‘wearable’ devices worn at the wrist for measurement of BP. If these devices aim to represent conventional brachial cuff BP, they may need to account for any differences in BP between the brachial and radial artery. Some authors suggest there are negligible differences between brachial and radial SBP and pulse pressure [24]; however, data from small invasive BP studies has shown brachial-to-radial SBP amplification exists [25,26]. We recently examined this issue using the largest invasive sample size to date (180 patients, mean age 61 years, 69% male) [27]. In this work invasive BP recordings were taken at the brachial and radial arteries. We found 43% of patients had radial SBP within ±5 mmHg of brachial SBP. Indeed, 46% of patients had radial SBP that was ≥5 mmHg higher than the brachial SBP, altogether indicating similar levels of individual variability in brachial-to-radial SBP amplification as the aorta-to-brachial arteries. This variability is exemplified in Figure 3, which shows gradual catheter pullback from the brachial-to-radial artery in two subjects, one with and one without brachial-to-radial SBP amplification [28]. Overall, the data from this study suggest that if biometric signals from the wrist are intended to estimate brachial SBP, the potential impact of brachial-to-radial SBP amplification on accuracy must be considered.
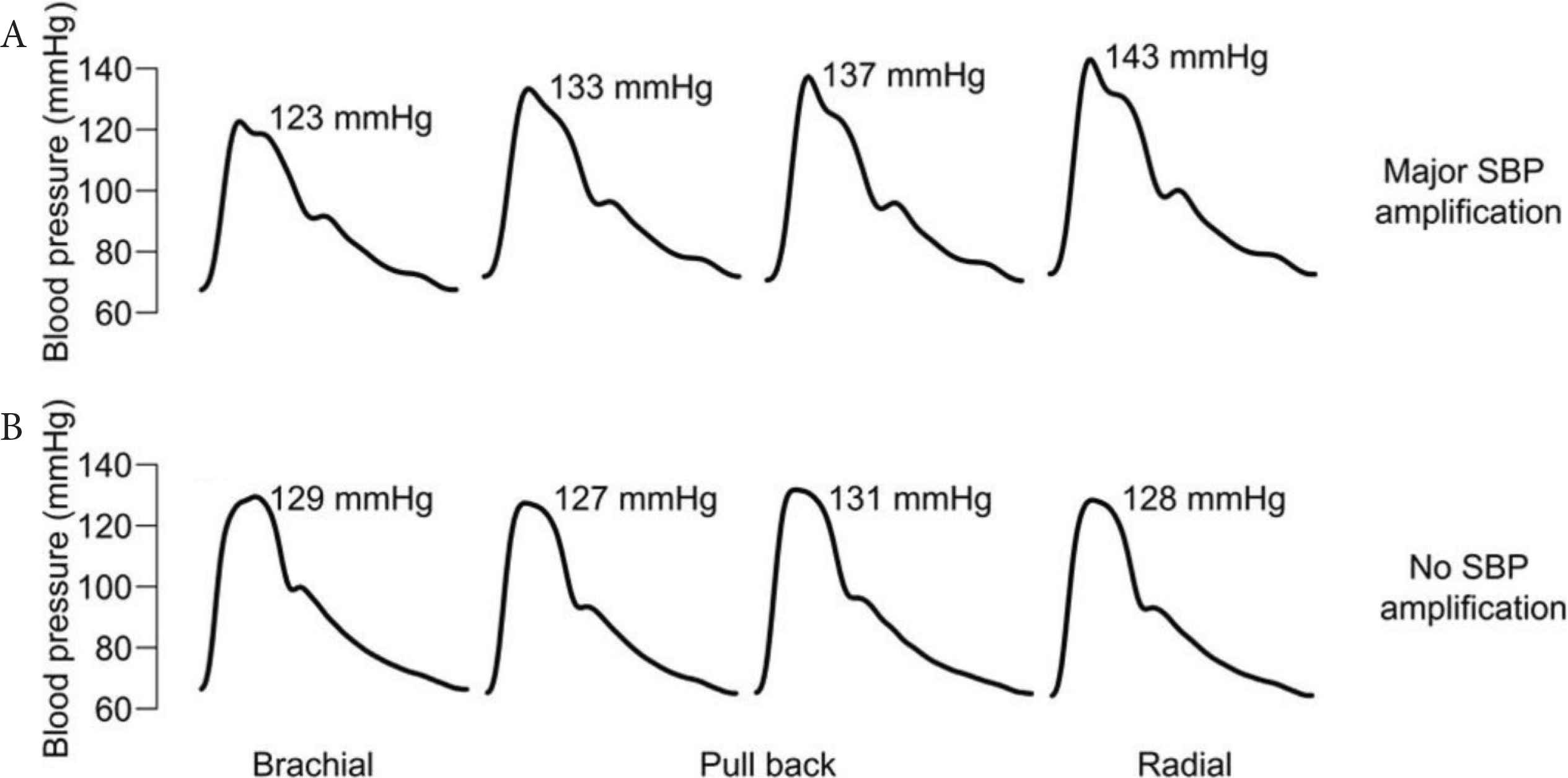
Variation in brachial-to-radial Systolic Blood Pressure (SBP) amplification observed in two subjects where fluid-filled catheters were withdrawn from the brachial-to-radial arteries at approximately 5 cm increments. The top subject exhibits major SBP amplification, while the bottom subject exhibits no SBP amplification. Figure adapted from Armstrong et al. [28] and republished in accordance with the AHA Copyright Transfer Agreement.
4. ACCURACY OF ESTIMATED CENTRAL AORTIC BLOOD PRESSURE DEVICES
The usefulness of estimated central (aortic) BP remains unresolved [29,30]. As alluded to earlier, there is a strong physiological rationale that central aortic BP may be a better predictor of cardiovascular risk than peripheral BP. However, there are myriad issues which plague the accuracy of devices that estimate central BP [31], including: (1) uncertainty on the best waveform calibration method [32]; (2) reliance on cuff BP for calibration of waveforms [33]; (3) failing to account for brachial-radial BP amplification (only applicable to radial tonometry methods) [34–37]; and (4) device-specific results due to different algorithms for the estimation of BP [31,32,38]. This final point has led to devices that estimate central BP being described as either ‘type I’ or ‘type II’ [31]. Type I refers to the devices that preserve the difference in central and brachial BP, meaning that BP amplification may be relatively accurate, despite substantial underestimation of central SBP (due to the calibration from cuff SBP underestimating the true underlying invasive waveform values) [32]. On the other hand, type II devices aim to estimate aortic BP as accurately as possible. These devices usually function by recalibration of the peripheral waveform with Mean Arterial Pressure (MAP) and DBP.
4.1. Implications of BP Amplification on Waveform Calibration
There is uncertainty on the most accurate calibration method to estimate central BP. In an attempt to resolve this issue, we conducted a study in which different calibration modes were tested using accurate, invasive BP to eliminate the problem of relying on inaccurate cuff BP calibration of peripheral waveforms [33]. In the study, 107 patients (mean age 62 years, 70% male) undergoing coronary angiography had invasive radial waveforms calibrated with either brachial SBP/DBP (the method popularised by the SphygmoCor system, AtCor Medical, Sydney, Australia) [39], or brachial MAP (area under the curve)/DBP. Estimated central BP was generated by the SphygmoCor CVMS (AtCor Medical, Sydney, Australia) via retrospective waveform processing using the software simulation mode. The results showed central BP estimates were significantly less accurate using the SBP/DBP calibration than MAP/DBP [40]. There were strong correlations of the difference in estimated and measured central aortic BP (i.e. accuracy) with aortic-to-brachial or brachial-to-radial SBP amplification. This indicates that further research to understand the causes of variable SBP amplification may assist to improve algorithms (e.g. generalised transfer function) used to estimated central BP. Any improvements to the algorithms must be coupled with an appropriate way to calibrate peripheral waveforms [33,36,37].
A potential solution for the accuracy problems that plague estimated central aortic BP was recently published [41]. The authors used a unique method to directly analyse the internal cuff pressure waveform from standard cuff inflation and deflation, instead of performing an additional inflation like most other devices on the market. The exact details are not known because this is a device-specific approach, but the work represents an important innovation for BP measurement and importantly passed the ARTERY Society validation protocol [31] when compared with invasive aortic SBP and DBP. Nevertheless, independent validation of device accuracy and studies on clinical relevance of the data from this device are needed.
5. FUTURE DIRECTIONS
Ongoing work to understand the physiological factors that contribute to the inaccuracy of cuff BP is needed. Such data will be critical for informing efforts to improve the accuracy of cuff BP. Some current work to improve the accuracy of BP measurement has involved deeper analysis of waveform information captured during oscillometry [41,42]. In the future methods that harness modern analytics, including artificial intelligence may help to improve BP measurement accuracy.
Aside from cuff techniques, it is also important to acknowledge that many cuff-less BP measurement approaches are under development [43,44]. The potential of cuff-less BP measurement is exciting, but measurement accuracy must not be overlooked [45–47]. There are already many cuff-less wrist wearables (activity tracking style devices) available online that purport to measure BP but have not undergone rigorous testing for accuracy [46,48].
6. SUMMARY
Cuff BP has been the cornerstone for the clinical management of BP for over 100 years. The data presented in this review does not dispute the evidence base on the associations of cuff BP with adverse clinical outcomes. Nevertheless, cuff BP is quite crude and our individual participant data meta-analyses have shown it is inaccurate in many people. We have also shown that a critical physiological factor associated with inaccuracy of BP measurement is SBP amplification. Taken together, our data suggest that improving the accuracy of BP measurement methods should be a research priority. Achieving this goal could lead to even better prediction of risk from BP measurements, leading to improved clinical practice and patient health outcomes through more accurate diagnosis and management of raised BP.
CONFLICTS OF INTEREST
The author declares no conflicts of interest.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Dean S. Picone PY - 2020 DA - 2020/06/27 TI - Accurate Measurement of Blood Pressure JO - Artery Research SP - 130 EP - 136 VL - 26 IS - 3 SN - 1876-4401 UR - https://doi.org/10.2991/artres.k.200624.001 DO - 10.2991/artres.k.200624.001 ID - Picone2020 ER -
