Canola oil decreases cholesterol and improves endothelial function in patients with peripheral arterial occlusive disease – a pilot study☆
Funding Sources: The study was partly funded by the Fondo Balli, Locarno, Switzerland.
- DOI
- 10.1016/j.artres.2008.02.001How to use a DOI?
- Keywords
- Peripheral arterial occlusive disease; Atherosclerosis; Polyunsaturated fatty acids; Endothelial function; Canola oil
- Abstract
Background: Dietary supplementation with omega-3 PUFAs has been shown to reduce cardiovascular morbidity and mortality. This pilot study investigated the effects of supplementation with plant-derived omega-3 and omega-6 PUFAs in patients with atherosclerosis.
Methods: Forty patients with PAD supplemented their usual diet with 2 tablespoons/day of canola oil (n = 20) or sunflower oil (n = 20), containing 2.24 g of α-linolenic acid or 16.24 g of linoleic acid, respectively, for 8 weeks. Laser Doppler flux (LDF), was assessed at rest and during reactive hyperaemia. Other measurements included parameters of heart rate variability (HRV), markers of plasmatic coagulation, fibrinolysis, platelet activation, inflammation, and lipid and homocysteine levels.
Results: Despite randomization, baseline values for LDF and HRV differed between the two groups. LDL-cholesterol decreased (from 2.74 ± 0.73 to 2.42 ± 0.65 mmol/L, p = 0.007) with canola oil but not with sunflower oil. The difference in the percent increase of LDF after ischemic challenge increased with canola oil from a median (25th; 75th percentiles) of 75.2% (48.6; 161.2) to 151.7% (117.8; 260.0) (p = 0.008) and with sunflower oil from 157.9% (125.4; 229.8) to 178.6% (127.3; 356.3) (p = 0.03), whereas a control group did show no change. HRV and other markers did not change.
Conclusions: Canola oil containing omega-3 PUFAs may confer cardiovascular protection by improving endothelial function and lowering LDL-cholesterol, but additional studies are warranted.
- Copyright
- © 2008 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Interventions that increase the amount of dietary omega-3 fatty acids have become an important issue in primary1 and secondary2 prevention of cardiovascular disease. Evidence from epidemiological studies and results from interventional trials have led to recommendations concerning the intake of omega-3 PUFAs for people with and without coronary heart disease.3 However, a recent metaanalysis cast doubts on the efficacy of such intervention:4 interventional study data is difficult to interpret due to the complexity of dietary interventions (e.g. the difficulty of attributing an effect to a single substance and the possible toxic effects of fish contaminants), and due to interactions/confounding factors (e.g. the interaction between marine and plant-based omega-3 PUFAs).5–7 Moreover, the mechanism(s) by which omega-3 PUFAs exert their biological effects are not fully understood, but may include hypolipemic, antiatherothrombotic, antiarrhythmic, and anti-inflammatory effects, as well as effects on endothelial function.8
Among the omega-3 PUFAs, plant-based ALA has been used in fewer interventional studies than marine-based omega-3 PUFAs such as EPA (C20:5n-3) and DHA (C22:6n-3).4 By desaturation and elongation ALA provides the substrates for vasodilating and antiaggregant prostaglandins and is a precursor for long-chain EPA and, to a lesser extent, for DHA;9 the increase in EPA levels following an ALA-rich diet, however, has been found to be modest.10 From epidemiological and interventional studies, however, it is unclear whether ALA confers benefits similar to marine-based omega-3 PUFAs for primary and secondary prevention of cardiovascular disease.11 The Western diet typically contains much more omega-6 than omega-3 PUFAs, which leads to a non-physiological omega-6:omega-3 balance and to production of interleukin-1, prostaglandins, and leukotrienes.2 Thus, plant-based LA, which is widely consumed in vegetable oils as a recommended substitute for saturated fats, is an omega-6 PUFA and a substrate for prostaglandins with inflammatory, vasoconstricting, and proaggregant properties.8,12
Surrogate markers commonly used to assess the effect of interventions on cardiovascular disease include markers of coagulation, platelet reactivity, inflammation, serum lipids, and homocysteine [Hcy].13–15 Available data on changes in these markers in response to increasing the amount of dietary omega-3 PUFAs are conflicting.6,11,16–22 Endothelial dysfunction, which is closely related to adverse cardiovascular events and is felt to be a sensitive marker of atherosclerosis,23 has also been assessed in the setting of dietary interventions with fatty acid supplementation.23,24 Moreover, the omega-3 PUFAs could exert a positive effect on arrhythmia, as has been suggested in the GISSI trial which showed a reduction in the number of sudden cardiac deaths.25
After considering all these data, we performed an interventional pilot study in patients with chronic PAD by adding canola (containing ALA) or sunflower oil (containing LA) to their usual diet. We analyzed surrogate endpoints, including parameters of atherothrombosis, fibrinolysis, inflammation, blood lipids, endothelial function, and heart rate variability.
Methods
Forty Caucasian patients, at least 50 years old with chronic PAD (defined as an ankle-brachial index <0.9 plus a duplex- or angiographically-based verification of a >50% stenosis or occlusion in a leg artery), were randomized in blocks of four (http://www.randomization.com). Exclusion criteria were acute, intercurrent illness; thromboangiitis obliterans; renal insufficiency (creatinine >130 μmol/L); acute myocardial infarction or stroke within 2 months; current oral anticoagulation medication; liver cirrhosis; and presence of an active malignant tumour. All participants were instructed not to change their habitual alimentation, which was supplemented in a blinded fashion with 2 tablespoons/day of vegetal oil taken without any additional serving. Group A (n = 20) received 2 tablespoons/day of canola oil, corresponding to a daily intake of 2.24 g of ALA (C18:3n-3; data from Sabo laboratory, Manno, Switzerland), and group B received 2 tablespoons/day of sunflower oil, corresponding to a daily intake of 16.24 g of LA acid (C18:2n-6). The oils were commercially available from Migros/Sabo oil (Manno, Switzerland), were packaged in similar bottles, and were filtered in a way that rendered them visually indistinguishable. Patients and doctors were blinded as to the contents of the bottles. The study period was 8 weeks, and compliance was assessed by the return of empty bottles. At baseline and at the end of the study, blood was drawn from an antecubital vein between 8 and 9 a.m. after 15 min of rest in a supine position. Blood was collected in a 10 ml plastic syringe (Monovette, Sarstedt, Nümbrecht, Germany) containing 1 ml 0.106 M trisodium citrate; blood samples were immediately placed on melting ice. Plasma was separated by cold centrifugation, and aliquots were stored at −70 °C until processing.
F 1 + 2 and TAT, both markers for thrombin generation, were measured by ELISA (Enzygnost F 1 + 2; Dade Behring, Marburg, Germany). DD, by-products of fibrinolysis, were also measured by ELISA (miniVidas by bioMérieux, Lyon, France). t-PA and PAI-1 were measured by ELISA (Hyphen Biomed, Neuville-sur-Oise France and Biopool, Umea, Sweden, respectively). Soluble CD40L [sCD40L], a marker of platelet activation, was determined by ELISA (Biosource, Camarillo, CA, USA). Plasma Hcy was determined by high-performance liquid chromatography and fluorescence detection. Ultrasensitive CRP was measured by nephelometry (Dade Behring, Marburg, Germany). Total cholesterol, as well as HDL- and LDL-cholesterol and TG, was measured with standard colorimetric methods (Roche Diagnostics, Zug, Switzerland).
Endothelial function was assessed by measuring skin blood flow after ischemic challenge using a laser Doppler flowmeter (PF 3, Perimed, Sweden), according to the method of Binggeli et al.26 Fasting patients were examined between 8:30 and 9:30 a.m. in a room with constant ambient temperature, after a 15-min resting period in a supine position. Reactive hyperaemia in skin microcirculation has been validated as a measure related to other established methods of endothelial function testing.26 Baseline blood flow was registered for 5 min using a probe fixed by a probe-holder close to the wrist. After 5 min, a cuff placed around the arm was left inflated to suprasystolic pressure for 4.5 min. After release of the cuff, reactive hyperaemic blood flow was registered, and the difference of blood flow between baseline and the mean flow between 30 and 60 s after release was expressed as a percentage of basal flow. A control group of 8 consecutive patients (6 men, 2 women, mean age 63.8 ± 12 years) with PAD and no dietary supplements was examined twice with LDF (with 3–8 weeks between measurements).
Heart rate variability analysis was conducted at the beginning of the study and 8 weeks later at the end of the study using a Mortara H-Scribe (Mortara Instruments Inc., Milwaukee, Wisconsin). Heart rate was recorded for 24 h, and the following time-domain parameters were obtained: %RR50, rmsSD, SDNN, and SDANN.
The protocol was approved by the local Ethics Committee, and informed, written consent was obtained from all participants.
Statistical analysis
Data are shown as mean ± SD or median (25th; 75th percentile). Differences between the groups at baseline were assessed with the t-test or with the rank sum test depending on the distribution of the data. Comparisons of data within the groups were done by paired t-test or signed rank test. A Chi-square test or a Fisher exact test was performed for comparing proportions. Linear correlation between LDL-cholesterol and LDF was sought for changes between values at baseline and those at the end of the study. Logistic regression analysis was done to look for a relationship between endothelial function and medication at baseline. A value of p < 0.05 (two-tailed) was considered statistically significant. We identified high responders for changes in LDF by using as cut-off points the mean plus 2 SD for the relative change in the assay of the control group.
Results
Compliance with oil ingestion was 100% in both groups, and the oils were well tolerated by all subjects. The characteristics and baseline measurements of the two groups were comparable, with the exception of the number of patients treated with ACE-inhibitors or AT-II antagonists, and except for endothelial function and heart rate variability (Table 1). Multiple logistic regression did not show interdependence between ACE-inhibitors or AT-II antagonists on one side and endothelial function and heart rate variability on the other (results not shown). Less patients had a double antiaggregation with aspirin and clopidogrel in the canola oil group compared with the sunflower oil group (2 out of 20 vs. 9 out of 20 patients, p = 0.03), and a weak association was found between double antiaggregation and LDF by logistic regression analysis (OR 1.01, 95% CI 1.001 to 1.02, p = 0.02).
| Canola | Sunflower | p | ||
|---|---|---|---|---|
| Age (mean ± SD) | 66.8 ± 8.1 | 63.7 ± 8.3 | NS | |
| m; f | 14; 6 | 13; 7 | NS | |
| Territory involved (n) | PAD | 20 | 20 | NS |
| CAD | 7 | 7 | NS | |
| CVD | 1 | 1 | NS | |
| Risk factor (n) | Smoker | 10 | 9 | NS |
| Arterial HT | 18 | 13 | NS | |
| DM | 5 | 6 | NS | |
| Medication (n) | Aspirin | 16 | 20 | 0.053 |
| Clopidogrel | 6 | 9 | NS | |
| Aspirin + Clopidogrel | 2 | 9 | 0.03 | |
| β-blocker | 5 | 8 | NS | |
| Statin | 20 | 17 | NS | |
| ACE-/AT II-inhibitor | 16 | 6 | 0.004 | |
| Calciumantagonist | 3 | 4 | NS | |
| Diuretic | 5 | 2 | NS | |
| Antidiabetic | 4 | 3 | NS | |
| Coagulation | F1 + 2 (pmol/L) | 217 ± 80 | 237 ± 135 | NS |
| TAT (μg/l) | 2.36 (1.8; 4.0) | 2.54 (2.3; 5.0) | NS | |
| DD (ng/ml) | 563 (422; 725) | 463 (371; 1023) | NS | |
| Fibrinolysis | t-PA (ng/ml) | 5.82 ± 1.6 | 5.36 ± 2.1 | NS |
| PAI-1 (ng/ml) | 25.6 ± 14.5 | 20.6 ± 10.3 | NS | |
| Platelets | sCD40L (ng/ml) | 0.37 (0.18; 0.67) | 0.45 (0.27; 1.5) | NS |
| Inflammation | CRP (mg/L) | 2.4 (1.1; 4.2) | 1.7 (1.2; 2.5) | NS |
| Homocysteine | Hcy (μmol/L) | 13.6 ± 4.0 | 13.2 ± 4.3 | NS |
| Lipids | Total cholesterol (mmol/L) | 4.73 ± 0.9 | 4.94 ± 1.3 | NS |
| HDL-cholesterol (mmol/L) | 1.44 ± 0.32 | 1.52 ± 0.46 | NS | |
| LDL-cholesterol (mmol/L) | 2.74 ± 0.73 | 2.77 ± 1.25 | NS | |
| TG (mmol/L) | 1.44 ± 0.55 | 1.56 ± 0.66 | NS | |
| Endothelial function | dLDF (%) | 75.2 (48.6; 161.2) | 157.9 (125.4; 229.8) | 0.02 |
| Heart variability | Heart rate (bpm) | 72 ± 11.7 | 72 ± 8.1 | NS |
| %RR50 (%) | 8 (1.5; 20.5) | 1 (0.5; 3.0) | 0.01 | |
| rmsSD (msec) | 63.6 ± 42.9 | 44.8 ± 30.0 | NS | |
| SDNN (msec) | 55.2 ± 25.3 | 37.4 ± 20.0 | 0.018 | |
| SDANN (msec) | 129.95 ± 44.0 | 102.75 ± 34.0 | 0.035 |
PAD, peripheral arterial disease; CAD, coronary artery disease; CVD, cerebro-vascular disease; arterial HT, arterial hypertension; DM, diabetes mellitus; NS, not significant.
Characteristics of study population at baseline
Markers of coagulation, fibrinolysis, and platelet activation did not change after canola or sunflower oil supplementation, nor did Hcy or CRP (Table 2).
| A | B | dMean (95% CI) | p | ||
|---|---|---|---|---|---|
| Canola | F1 + 2, pmol/L | 217 ± 80 | 221 ± 93 | 4 (−28–19) | NS |
| TAT, μg/l | 3.2 ± 1.9 | 2.7 ± 1.0 | 0.5 (−0.4–1.3) | NS | |
| DD, ng/ml | 640 ± 365 | 651 ± 406 | 11 (−101–79) | NS | |
| tPA, ng/ml | 5.82 ± 1.59 | 5.95 ± 1.74 | 0.13 (−0.69–0.43) | NS | |
| PAI-1, ng/ml | 25.6 ± 14.5 | 26.2 ± 10.6 | 0.57 (−5.9–4.7) | NS | |
| sCD40L, ng/ml | 0.37 (0.18; 0.67) | 0.4 (0.17; 0.64) | NA | NS | |
| Hcy, μmol/L | 13.6 ± 4.0 | 13.9 ± 3.7 | 0.2 (−1.6–1.1) | NS | |
| CRP, mg/L | 2.4 (1.1; 4.2) | 1.6 (1.0; 5.7) | NA | NS | |
| Sunflower | F1 + 2, pmol/L | 237 ± 135 | 236 ± 132 | 1.0 (−26–28) | NS |
| TAT, μg/l | 3.3 ± 2.1 | 3.6 ± 2.7 | 0.3 (−1.5–0.8) | NS | |
| DD, ng/ml | 717 ± 507 | 592 ± 372 | 125 (−29–279) | NS | |
| tPA, ng/ml | 5.4 ± 2.1 | 5.4 ± 2.4 | 0.05 (−0.68–0.57) | NS | |
| PAI-1, ng/ml | 20.7 ± 10.3 | 18.4 ± 9.2 | 2.3 (−0.07–4.6) | NS | |
| sCD40L, ng/ml | 0.45 (0.27; 1.49) | 0.57 (0.26; 1.45) | NA | NS | |
| Hcy, μmol/L | 13.2 ± 4.3 | 12.5 ± 3.4 | 0.7 (−0.4–1.9) | NS | |
| CRP, mg/L | 1.7 (1.0; 5.7) | 1.7 (0.9; 4.7) | NA | NS |
A is for baseline, B, after 8 weeks oil supplementation. Values are given as mean ± SD except for sCD40L and CRP, where values are median (25th; 75th percentile). p denotes p-values for comparison between A and B.
Influence of PUFA supplementation on parameters of atherothrombosis
Canola oil significantly reduced total cholesterol as well as LDL-cholesterol, although most patients were on statins and already had low values at baseline; sunflower oil had no effect on cholesterol concentrations. TG levels did not change in either group (Table 3).
| A | B | dMean (95% CI) | p | ||
|---|---|---|---|---|---|
| Canola | Tot. cholesterol, mmol/L | 4.73 ± 0.89 | 4.42 ± 0.89 | 0.32 (0.06–0.57) | 0.017 |
| HDL-C, mmol/L | 1.44 ± 0.32 | 1.46 ± 0.4 | 0.02 (−0.11–0.08) | NS | |
| LDL-C, mmol/L | 2.74 ± 0.73 | 2.42 ± 0.65 | 0.31 (0.1–0.53) | 0.007 | |
| TG, mmol/L | 1.32 (1.06; 1.79) | 1.29 (1.03; 1.83) | NA | NS | |
| Sunflower | Tot. cholesterol, mmol/L | 4.94 ± 1.28 | 4.87 ± 1.46 | 0.07 (−0.26–0.4) | NS |
| HDL-C, mmol/L | 1.52 ± 0.46 | 1.63 ± 0.6 | 0.11 (−0.25–0.03) | NS | |
| LDL-C, mmol/L | 2.77 ± 1.25 | 2.71 ± 1.31 | 0.06 (−0.2–0.31) | NS | |
| TG, mmol/L | 1.44 (1.0; 2.1) | 1.24 (0.96; 1.76) | NA | NS |
A denotes baseline; B, after 8 weeks’ oil supplement. Values are mean ± SD or median (25th; 75th percentile).
Concentration of lipids at baseline and after oil supplementation
LDF of controls and of patients in the canola oil group were not different, whereas endothelial function was better preserved in the sunflower oil group in spite of randomization (Fig. 1). Postischemic LDF increased with both oils; the comparison was statistically highly significant for canola oil (p = 0.008) but less so for sunflower oil supplementation (p = 0.03) (Table 4). Comparing both groups with a control group, the median values of differences of % LDF variation were significantly different for the canola oil group (p = 0.016) but not for the sunflower oil group (p = 0.071); in contrast, both groups did not differ statistically from each other, although the LDF increase in individual patients in the canola oil group was more consistent. In fact, there were 5 high responders in the canola oil group with a difference of %LDF variation >500%, compared to none in the sunflower oil group (p = 0.047 by Fisher exact test). The correlation coefficient for LDF variations at 2 time points in the control group was 0.9. Changes in LDL-cholesterol did not correlate with changes in LDF in the canola oil group.
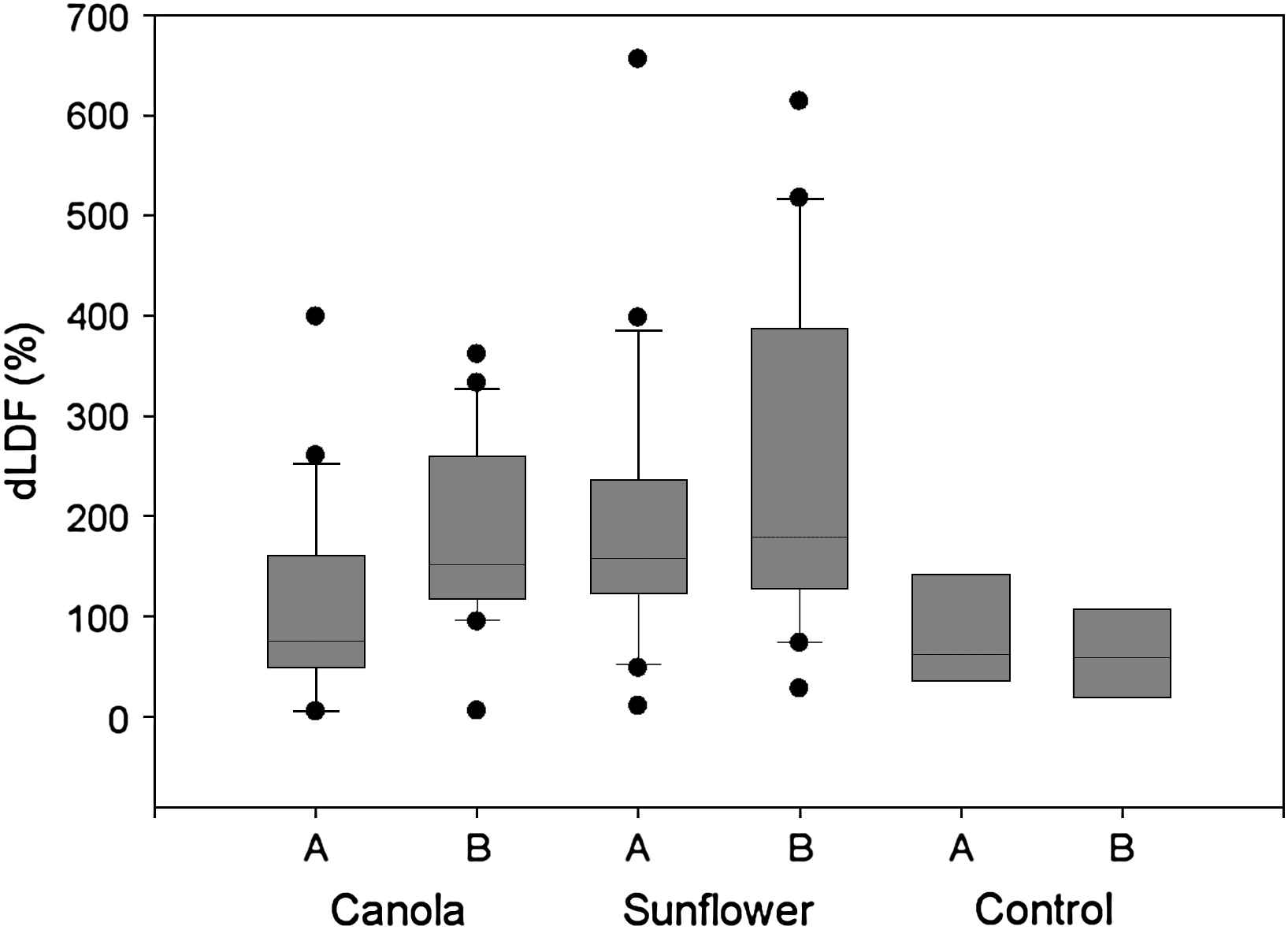
Box plot of difference of postischemic flow (dLDF) at baseline (A) and at the end of the study (B). Values are significantly different for canola oil A vs. B (p = 0.008), sunflower oil A vs. B (p = 0.03), canola oil vs. sunflower oil at baseline (p = 0.02), sunflower oil vs. control at baseline (p = 0.02), but not between canola oil vs. control at baseline (p = 0.78) and control A vs. B (p = 0.8).
| A | B | p | ||
|---|---|---|---|---|
| Canola (n = 20) | dLDF (%) | 75.2 (48.6; 161.2) | 151.7 (117.8; 260.0)* | 0.008 |
| Sunflower (n = 20) | dLDF (%) | 157.9 (125.4; 229.8) | 178.6 (127.3; 356.3)* | 0.03 |
| Control (n = 8) | dLDF (%) | 61.6 (36.9; 118.8) | 59.2 (24.9; 103.7) | NS |
LDF denotes laser doppler flow; A, baseline, B, after 8 weeks. Values are median (25th; 75th percentiles).
p < 0.01 for comparison between control and either group.
Increase of postischaemic LDF between baseline and after 8 weeks’ of oil supplementation
Indices of heart rate variability are an expression of parasympathetic tone. %RR50 increased in more patients in the canola oil group than in the sunflower oil group (10 out of 20 and 5 out of 20, respectively), but the difference by chi-square test was not significant (p = 0.23). rmsSD changed from 63.6 ± 42.9 ms to 61.2 ± 37.1 ms for the canola oil (p = 0.72) and from 44.8 ± 30.0 to 36.8 ± 21.1 ms for the sunflower oil group (p = 0.19); differences between the 2 groups were 18.9 ms (95% CI −4.9–42.6; p = 0.12) at baseline and 24.4 ms (95% CI 4.7–44.1; p = 0.017) after 8 weeks. SDNN and SDANN were different between the two groups at baseline; there was no effect of oil supplementation within either group (Table 5).
| Variables | Canola oil group | Sunflower oil group | ||||
|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | |
| HR (bpm) | 72.0 ± 11.7 | 72.5 ± 11.9 | 0.68 | 72.4 ± 8.1 | 72.0 ± 8.0 | NS |
| %RR50 (%) | 14.2 ± 17.4 | 16.0 ± 17.6 | 0.25 | 4.2 ± 7.2† | 3.11 ± 4.2‡ | NS |
| rmsSD (msec) | 63.6 ± 42.9 | 61.2 ± 37.1 | 0.72 | 44.75 ± 30.0 | 36.8 ± 21.1* | NS |
| SDNN (msec) | 55.2 ± 25.3 | 54.3 ± 23.5 | 0.79 | 37.4 ± 19.9* | 35.9 ± 14.4† | NS |
| SDANN (msec) | 129.9 ± 44.0 | 126.7 ± 32.8 | 0.63 | 102.75 ± 34.0* | 106.5 ± 36.2* | NS |
HR is for heart rate.
p: p-value for comparison within either group before and after the experimental diet.
p-value <0.05 for comparison between the groups.
p-value <0.01 for comparison between the groups.
p-value <0.001 for comparison between the groups.
Influence of oil supplementation on parameters of heart rate variability
Discussion
This study found that a usual diet supplemented daily with 2 tablespoons of canola oil for 8 weeks, which corresponds to 2.24 g of ALA, improved endothelial function in patients with stable chronic PAD and reduced total and LDL-cholesterol, even though the subjects were already being treated extensively with antiaggregants, statins, and vasoactive substances. Similar supplementation with 2 tablespoons of sunflower oil, containing 16.24 g of LA, resulted in no change in serum lipid concentrations; endothelial function was improved to a lesser extent. Neither intervention had an effect on markers of coagulation, fibrinolysis, platelet activation, inflammation, or Hcy. There was no significant change in parameters of heart rate variability and thus no significant change in the sympathovagal balance, although we noted a trend in favour of ALA.
An important limitation of this study is that in spite of randomization the two groups differed at baseline in heart rate variability parameters and endothelial function indicators. Unmatched medications at baseline may explain the better postischemic flow in the sunflower oil group: in fact, we found a weak association with double antiaggregation and endothelial function. There was no correlation between ACE- or AT II inhibitors, known to interfere with heart rate variability and endothelial responsiveness,27–29 and endothelial function and HRV parameters. Thus, the partial lack of matching at baseline for the two groups does not allow to conclude for different effects of the two oils on endothelial function; the positive effect on postischemic flow especially with canola oil compared with a control group of consecutive, non-selected patients, however, points to a possible positive influence of (omega-3) PUFA on endothelial function.
The duration of this study may have been too short for some of the parameters studied; in similar clinical trials, however, experimental groups often show a difference from control groups after only a few weeks.30 In addition, a study of several months’ duration may face problems with compliance,31 which in our study was excellent.
The results from studies investigating a cholesterol-lowering effect of ALA have been equivocal. In a recent review of epidemiological and clinical data ALA is suggested to constitute a major cardioprotective nutrient,32 whereas almost contemporaneously a workshop concluded that ALA supplementation did not result in consistent beneficial effects on serum lipids.33 The findings of this study, however, point to a favourable effect of ALA-rich canola oil on LDL-cholesterol, and are in line with a recent crossover trial which found a similar reduction of total and LDL-cholesterol by a diet supplemented with nuts compared to a diet supplemented with canola oil enriched cereal in healthy subjects.34 The results of our study extend these findings to cardiovascular patients extensively treated with cholesterol-lowering drugs and could therefore have an impact on guidelines for secondary prevention in atherosclerotic disease.
Endothelial dysfunction is an early pathologic marker in subjects with hypercholesterolemia or the metabolic syndrome and is a hallmark of patients with clinically overt atherosclerosis.23,35,36 The assessment of endothelial function can be invasive or non-invasive; among the non-invasive methods, ultrasound-based measurement of FMD in the brachial artery is most widely used.37 While FMD is mainly mediated by the release of endothelial NO, other tests evaluating reactive hyperaemia, such as the measurement of skin blood flow by laser Doppler, can assess the release of vasoactive prostaglandins.26 Omega-3 supplementation has been shown to improve the dilation of the brachial artery;8 this was found in healthy subjects with fish oil but not with plant-derived omega-6 or omega-3 oils, as assessed by LDF.24 The results of our study are in line with those of Ros et al., who found improved endothelial function using FMD of the brachial artery in hypercholesterolemic subjects after 8 weeks of a ALA-containing walnut/Mediterranean diet crossover trial; interestingly, the authors also found a decrease in total and LDL-cholesterol.30
The mechanisms by which an ALA-rich diet influence endothelial function are not clear. It is well established that aggressively lowering cholesterol positively influences endothelial function.38 However, lowering LDL-cholesterol by 11% when it is already in a low range makes it unlikely that the only mechanism involved is a lipid-lowering mechanism. Moreover, changes in cholesterol did not correlate with changes in LDF in this study. There are arguments in favour of the prostaglandin hypothesis as the mechanism by which ALA causes improvement in endothelial function. First, prostaglandins were shown to play a role in postischemic skin hyperaemia.26 Second, in our study the sunflower oil containing omega-6 PUFAs also resulted in an endothelial response compared to the control group, despite its neutral effect on blood lipids. This could be explained by a major synthesis of prostacyclin. Prostacyclin, a series 2 prostaglandin produced from omega-6 arachidonic acid, may be present in a relatively higher amount in patients whose cyclooxygenase is inhibited by aspirin and who thus form lower amounts of the vasoconstrictor thromboxane A2.8 Moreover, a diet rich in walnuts (containing ALA) has been shown to improve endothelial function30,39 by a mechanism that was felt to include omega-3 PUFA but not antioxidants.39 Taken together, the data in our study argue in favour of ALA-derived prostaglandins driving endothelial function improvement.
We conclude that canola oil may confer cardiovascular protection, most likely by the dual mechanisms of ameliorating endothelial function and lowering LDL-cholesterol. Sunflower oil may improve endothelial function, too, but has no effect on cholesterol. Our preliminary results should motivate further studies to assess the impact of plant-derived PUFA’s on cardiovascular parameters.
Acknowledgements
The study was partly funded by the Fondo Balli, Locarno, Switzerland.
Abbreviations
- %RR50
the percent difference between adjacent RR intervals that were greater than 50 msec;
- ALA
α-linolenic acid;
- DHA
docosapentaneoic acid;
- DD
D-dimers;
- EPA
eicosapentaneoic acid;
- F1 + 2
prothrombin fragment 1 + 2;
- FMD
flow-mediated dilation;
- GISSI
gruppo italiano per lo studio della sopravvivenza nell’infarto miocardio;
- LA
linoleic acid;
- LDF
laser Doppler flow;
- NO
nitric oxide;
- PAD
peripheral artery occlusive disease;
- PAI-1
plasminogen activator inhibitor-1;
- PUFA
polyunsaturated fatty acid;
- rmsSD
the square root of the mean of the sum of the squares of differences between adjacent RR intervals;
- SDANN
the standard deviation of the normal RR intervals averaged over 5 min intervals during the monitoring period;
- SDNN
the standard deviation of all normal RR intervals observed over the monitoring period;
- TAT
thombin-antithrombin complex;
- t-PA
tissue-type plasminogen activator.
References
Cite this article
TY - JOUR AU - Hans Stricker AU - Francesca Duchini AU - Marco Facchini AU - Giorgio Mombelli PY - 2008 DA - 2008/03/12 TI - Canola oil decreases cholesterol and improves endothelial function in patients with peripheral arterial occlusive disease – a pilot study☆ JO - Artery Research SP - 67 EP - 73 VL - 2 IS - 2 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2008.02.001 DO - 10.1016/j.artres.2008.02.001 ID - Stricker2008 ER -
