Combination therapy in hypertension: From effect on arterial stiffness and central haemodynamics to cardiovascular benefits☆
This article was presented as lecture in the symposium on “Arterial stiffness: a translational approach” at the ARTERY 14 Congress in Maastricht, The Netherlands (October 9–11, 2014).
- DOI
- 10.1016/j.artres.2016.02.005How to use a DOI?
- Keywords
- Aortic stiffness; Pulse wave velocity; Wave reflections; Central haemodynamics; Hypertension; Angiotensin converting enzyme inhibitors; Calcium channel blockers
- Abstract
Measures of arterial aging have the potential to improve risk prediction beyond traditional risk scores. Such biomarkers that fulfil most, or some of the strict criteria of a surrogate end-point are aortic stiffness (IIa level of recommendation in European Guidelines and Position Papers) and central haemodynamics (IIb level of recommendation). Early intervention towards improving aortic elastic properties acquires particular importance since evidence suggests that arterial stiffening may occur before the onset of hypertension. Part of the beneficial effects of antihypertensive treatment in risk reduction may be mediated through improvement in aortic stiffness and central haemodynamics. However, not all antihypertensive drugs affect aortic stiffness and central haemodynamics in a similar way. Angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARB) and calcium channel blockers (CCB) have beneficial effects on such parameters. Meta-analytical approaches have shown that ACE inhibitors reduce mortality in hypertension, whereas ARBs do not exhibit such a benefit. Furthermore, ACE inhibitors have been shown to reduce the risk of coronary artery disease, and CCBs to reduce the risk of stroke independently of blood pressure reduction. Combining an ACE inhibitor with a CCB has the potential to reduce cardiovascular risk (synergy at the clinical level) by reducing aortic stiffness and improving central haemodynamics (synergy at the vascular level).
- Copyright
- © 2016 Published by Elsevier B.V. on behalf of Association for Research into Arterial Structure and Physiology.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Predicting cardiovascular risk in hypertension
Risk scores such as the SCORE and the Framingham are of paramount importance for adapted preventive strategies in clinical decision-making. However, at instances a significant gap exists between predicted and actual event rates, leading to under- and over-prediction. Additional tools to further stratify the risk of patients at an individual level are biomarkers. A surrogate endpoint is a biomarker that is intended as a substitute for a clinical endpoint. In order to be considered as a surrogate endpoint of cardiovascular events, a biomarker should satisfy several criteria, such as proof of concept, prospective validation, incremental value, clinical utility, clinical outcomes, cost-effectiveness, ease of use, methodological consensus, and reference values.1 Conceptually, arterial biomarkers gauge vascular aging. According to this concept, individual risk factors may fluctuate and at the time of risk assessment they may not truly reflect their impact on arterial wall, whereas arterial biomarkers have the potential to measure their accumulated damage on the arterial wall over a long period of time.2
The addition of a vascular biomarker adds to risk prediction modestly, yet significantly beyond classical risk factors and may be useful in patients classified as having intermediate cardiovascular (CV) risk and in whom there is a therapeutic dilemma. Nevertheless, it is still unclear whether a specific vascular biomarker is superior over the others. However, some fulfil most, and others fulfil some of the criteria. In the first category belongs aortic stiffness (IIa level of recommendation) and in the second central haemodynamics (IIb level of recommendation).1,3
Aortic stiffness
Arteriosclerosis is a different term to atherosclerosis. Arterial stiffening results primarily from arteriosclerosis (principally a disease of the media, related to normal or accelerated aging) rather than from atherosclerosis (principally a disease of the intima, affecting the vessel in a patchy and not uniform manner). The aorta is a major vessel of interest when determining regional arterial stiffness, since the thoracic and abdominal aorta make the largest contribution to arterial buffering. cfPWV (carotid-femoral pulse wave velocity), i.e. the velocity of the pulse as it travels from the heart to the carotid and the femoral artery, remains the most commonly used non-invasive method and is considered as the “gold standard” for the assessment of aortic stiffness.4,5
The predictive value of aortic stiffness has been convincingly demonstrated in studies spanning from high-risk groups, such as end-stage renal disease, to the general population.6–15 Importantly, aortic stiffness is predictive even after adjustment for the FRS7 or SCORE,16 attesting to its added value to a combination of CV risk factors. In the latter study, risk of CV death was associated with LV hypertrophy, atherosclerotic plaques and cf-PWV >12 m/s, independently of SCORE risk stratification in a population-based sample of 1968 subjects that were followed for a median of 12.8 years. Importantly, cf-PWV had the strongest prognostic importance compared with other markers of subclinical organ damage in subjects with SCORE <5% (Fig. 1).16
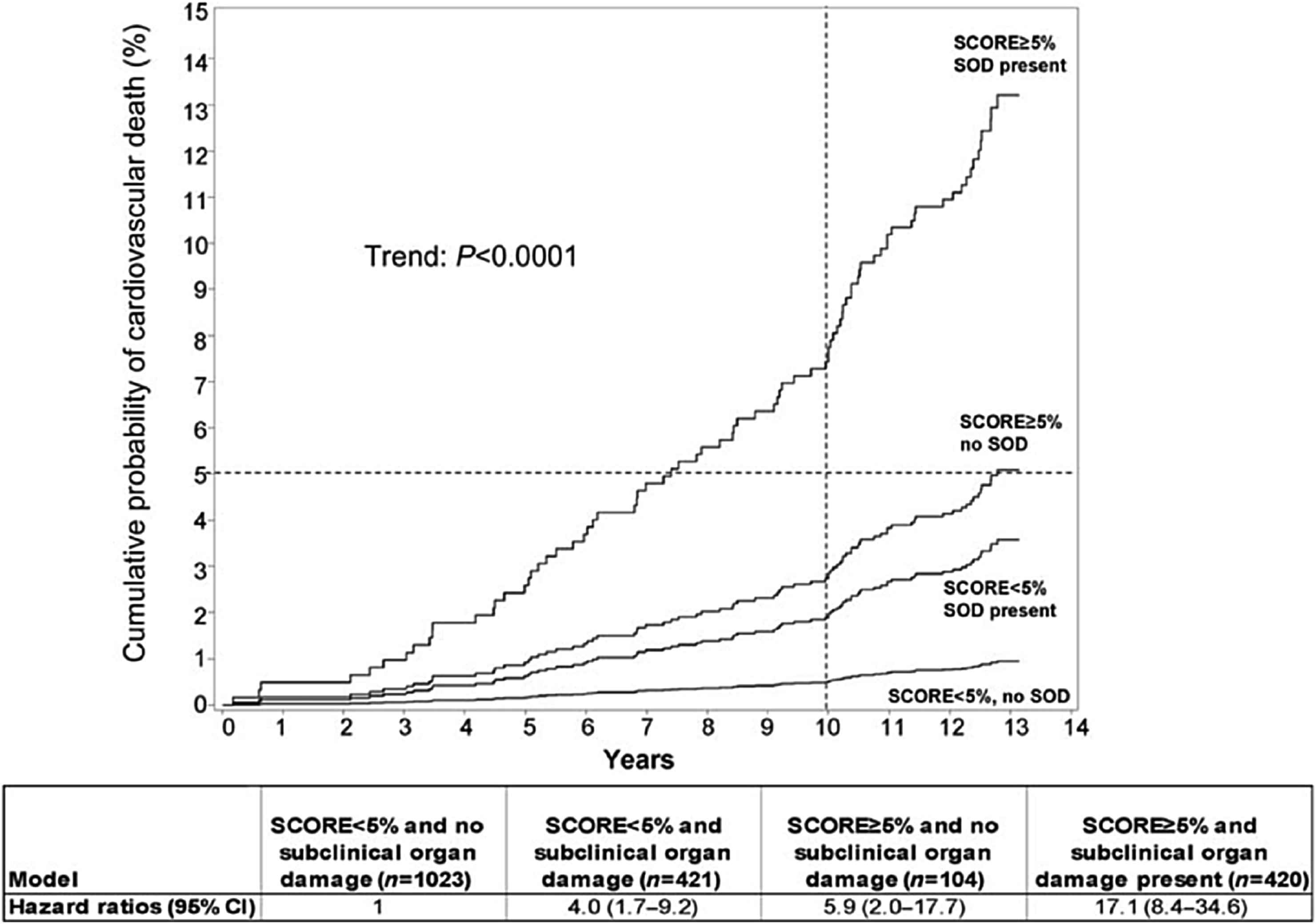
The cumulative probability (%) and hazard ratios of cardiovascular death in subgroups, according to SCORE and presence of sub-clinical organ damage. Dotted lines denote the 10 years 5% cumulative probability of cardiovascular death. Subjects with SCORE, 5% and no subclinical organ damage were the reference group. Abbreviation: SOD, subclinical organ damage. Modified from Sehestedt et al.16
In 2010, we gauged the effect of aortic stiffness (assessed by c-fPWV) on events in a systematic meta-analysis of 17 longitudinal studies in 15,877 subjects over a 7.7 year period, and we reported an update analysis in 2014 (27 longitudinal studies, 22,611 subjects); increased arterial stiffness was linked to a twofold increase in CV events and mortality, as well as all-cause mortality for subjects with high versus low aortic PWV (Fig. 2).17,18 An increase in aortic PWV by 1 m/s corresponds to an age-, sex-, and risk factor-adjusted risk increase of 14% in total CV events, 15% in CV mortality, and 15% in all-cause mortality. An increase in aortic PWV by 1 standard deviation was associated with respective increases of 47%, 47%, and 42%. Furthermore, in 2014, an individual data meta-analysis confirmed these results and showed that CV events increased by 30% per 1-SD increase of cfPWV (95% CI: 1.18–1.43) after adjustment for traditional risk factors19 (Fig. 2). Interestingly, the independent association of PWV with all-cause mortality merits attention, as it indicates that the role of arterial stiffness extends beyond diseases of the CV system. Of paramount importance is that PWV has the ability to change (reclassify) a person’s risk in a clinically meaningful way and move them into a different risk category (clinical utility criterion of a surrogate end-point).13,14,18,20 The 5-year overall NRI for coronary heart disease and stroke in intermediate risk individuals was 14.8% and 19.2% respectively in the individual data metanalysis.17 Reference values have been provided for PWV by the Reference Values for Arterial Stiffness in 1455 healthy subjects and a larger population of 11,092 subjects with CV risk factors,21 as well as for children and teenagers.22
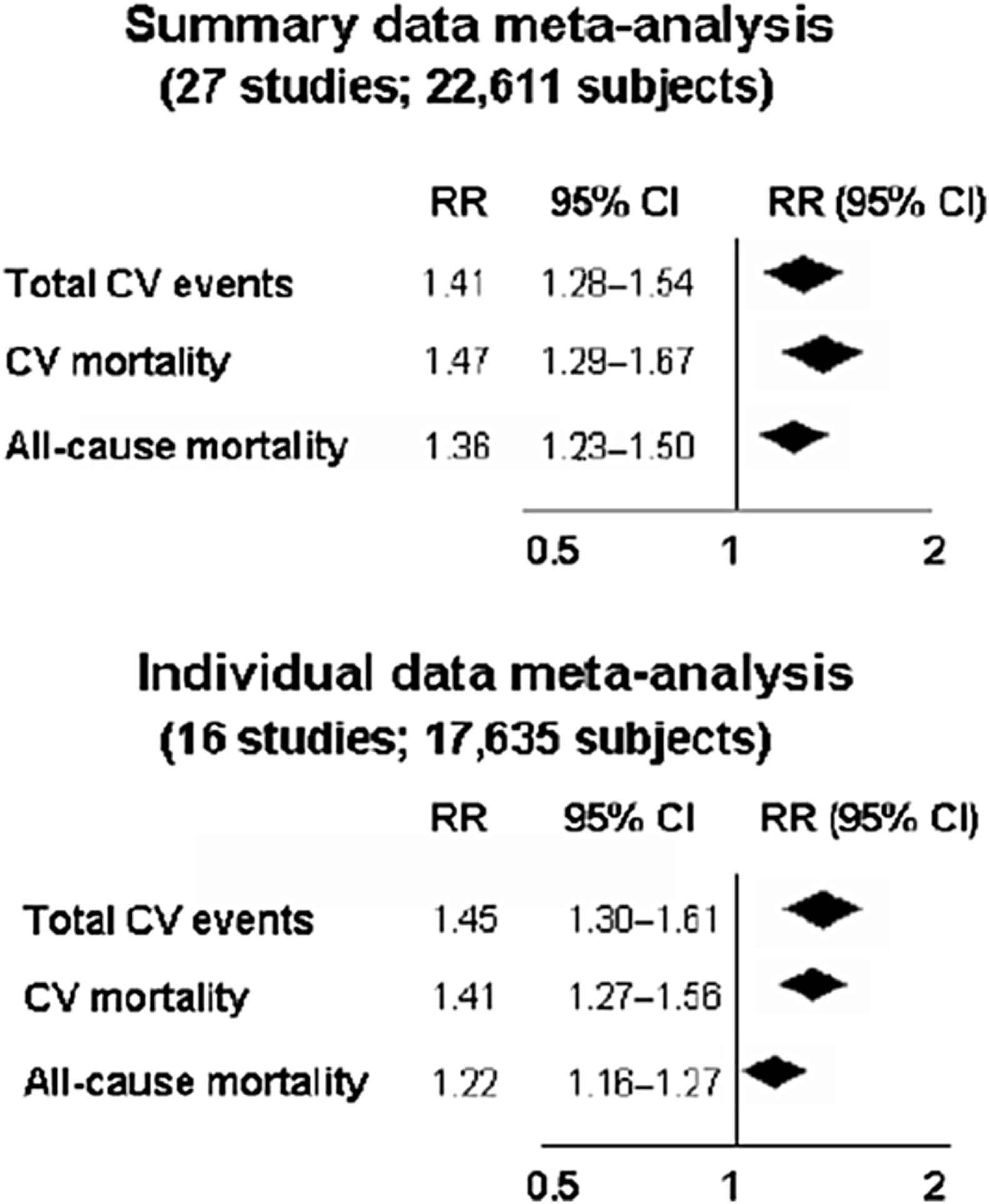
Relative risks (RR) and 95% CIs for a 1-SD increase in aortic PWV and total CV events, CV mortality, and all-cause mortality according to summary-data meta-analysis (updated analysis of Ref. 6 including 10 new studies [that reported risk for a 1-SD increase in aPWV in a literature search until September 2013] to a total of 27 studies, top panel) and individual data meta-analysis (Ref. 17, 16 studies, Ben-Shlomo et al. J Am Coll Cardiol 2014, bottom panel). The diamonds and their width represent the pooled RRs and the 95% CIs, respectively. Modified from: Vlachopoulos et al. J Am Coll Cardiol. 2014 Feb 25;63(7):647–9.17
Effects of different antihypertensive agents on arterial stiffness are described elsewhere.23
Central haemodynamics-wave reflections
Central (aortic, carotid) pressure is invariably lower from peripheral blood pressure (BP). Although diastolic and mean arterial BP are relatively constant along the arterial tree, systolic BP increases towards the periphery (amplification), which is mainly due to arterial stiffness increase moving away from the heart.1,24–27 While brachial BP predicts cardiovascular events, central BP by representing the “true” pressure of target organs damaged by high BP, central BP has, at least conceptually, the potential to predict cardiovascular outcomes better than the brachial BP.
Office central pulse pressure (PP) is associated with target organ damage of the macrocirculation (heart, carotid arteries).24–27 A circadian fluctuation of central systolic BP exists, exhibiting lower PP amplification during the night.28–31 Aortic 24-h PP is associated more closely than brachial 24-h PP with left ventricular mass, left ventricular diastolic dysfunction and common carotid artery hypertrophy.30 Central BP has been shown to be marginally significantly associated with adverse outcome, including mortality, in a recent meta-analysis (Fig. 3),32 while central BP or wave reflection indices were associated with adverse outcome in individual trials.33–40 Confirmation regarding stroke has been provided by preliminary results from an individual data meta-analysis.41 An important step towards clinical implementation was the recent publication of Reference values of central pressures by the Arterial Measurements Collaboration.42
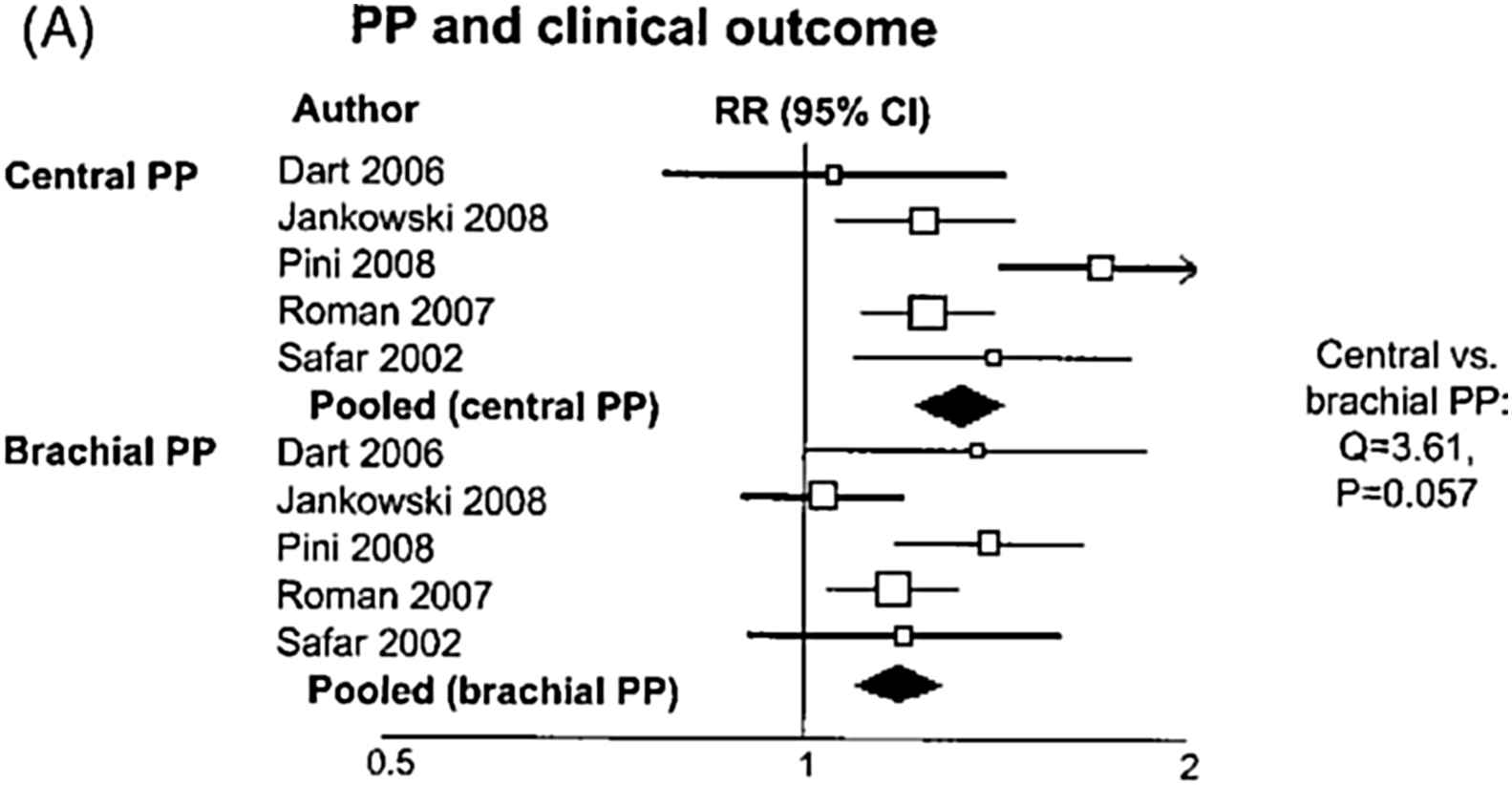
Relative risk (RR) and 95% confidence interval (CI) of clinical events for a 1 standard deviation increase in pulse pressure according to the site of measurement (central vs. brachial). Boxes represent the RRs and lines represent the 95% CI for individual studies. The diamonds and their width represent the pooled RR and the 95% CI, respectively. Modified from Vlachopoulos et al.32
Not all antihypertensive drugs have the same effect on central pressures and this is presented in detail elsewhere23,43 (Table 1). It is important to note that treatment may have differential effects on brachial compared with central BP,39,43–46 while organ damage regression is better associated with central than peripheral PP reduction.47 Interestingly, a single study showed that central BP-guided management was associated with fewer medication use without adverse effects on left ventricular mass, aortic stiffness, or quality of life, compared to brachial BP-guided management.48 Studies with hard endpoints are awaited.
| Class of antihypertensive drug | Decrease in central BP compared to decrease in peripheral BP | Level of evidence |
|---|---|---|
| Diuretics | cBP = pBP or cBP < pBP | More data are needed |
| b-Blockers | cBPs < pBPs | Compelling |
| ACEis | cBPs > pBPs | Convincing |
| ARBs | cBPs > pBPs or cBP = pBP | More data are needed |
| CCBs | cBPs > pBPs | More data are needed |
| Nitrates | cBPs > pBPs | Convincing but more data are needed |
ACEi: angiotensin converting enzyme inhibitors; ARB: angiotensin receptor blockers; CCB: calcium channel blockers (dihydropyridines). Modified from Ref 43.
Summary of the available evidence on the effect of antihypertensive classes of drugs on central blood pressure (cBP) lowering capacity beyond peripheral (pBP) lowering.
Intense CV risk factor reduction
Cardiovascular risk factors interact with each other and moderate reductions in several risk factors can be more effective than major reductions in one.49,50 Many CV risk factors are associated with increased arterial stiffness and/or wave reflections, including obesity, smoking, hypertension, hypercholesterolaemia, impaired glucose tolerance, metabolic syndrome, diabetes (types 1 and 2).1,4,27 Some risk factors, such as age and BP, are more important than others when assessing arterial stiffness.1,4,51 Given the prognostic role of PWV and central haemodynamics for CV outcomes, risk reduction by controlling of RF may be mediate through improvement in arterial function.
Benefits of combinations: synergy at the clinical level
Combining antihypertensive agents has numerous benefits. Adding a drug from another class is 5 fold more effective than doubling the dose of the first drug and BP targets are achieved faster52; complications are reduced53 and adherence is increased.54 Ultimately better adherence leads improved CV protection.55,56
Combination of a renin–angiotensin–aldosterone system (RAAS) blocker and a calcium channel locker (CCB) is very effective in controlling BP. Further, there is convincing evidence that angiotensin converting enzyme (ACE) inhibitors and CCBs may be a particularly useful combination in terms of clinical synergy.57–60 ACE inhibitors have been shown to reduce the risk of coronary artery disease (CAD), and CCBs to reduce the risk of stroke independently of BP reduction (Fig. 4).59 In addition, ACE inhibitors appear to have an added advantage compared with ARBs of reducing the risk of mortality in hypertension as meta-analyses have shown.57 Side effects can also be reduced: adding ACE inhibitor to CCB reduced peripheral oedema by over half, while this reduction is 21% when an ARB is combined with a CCB.53
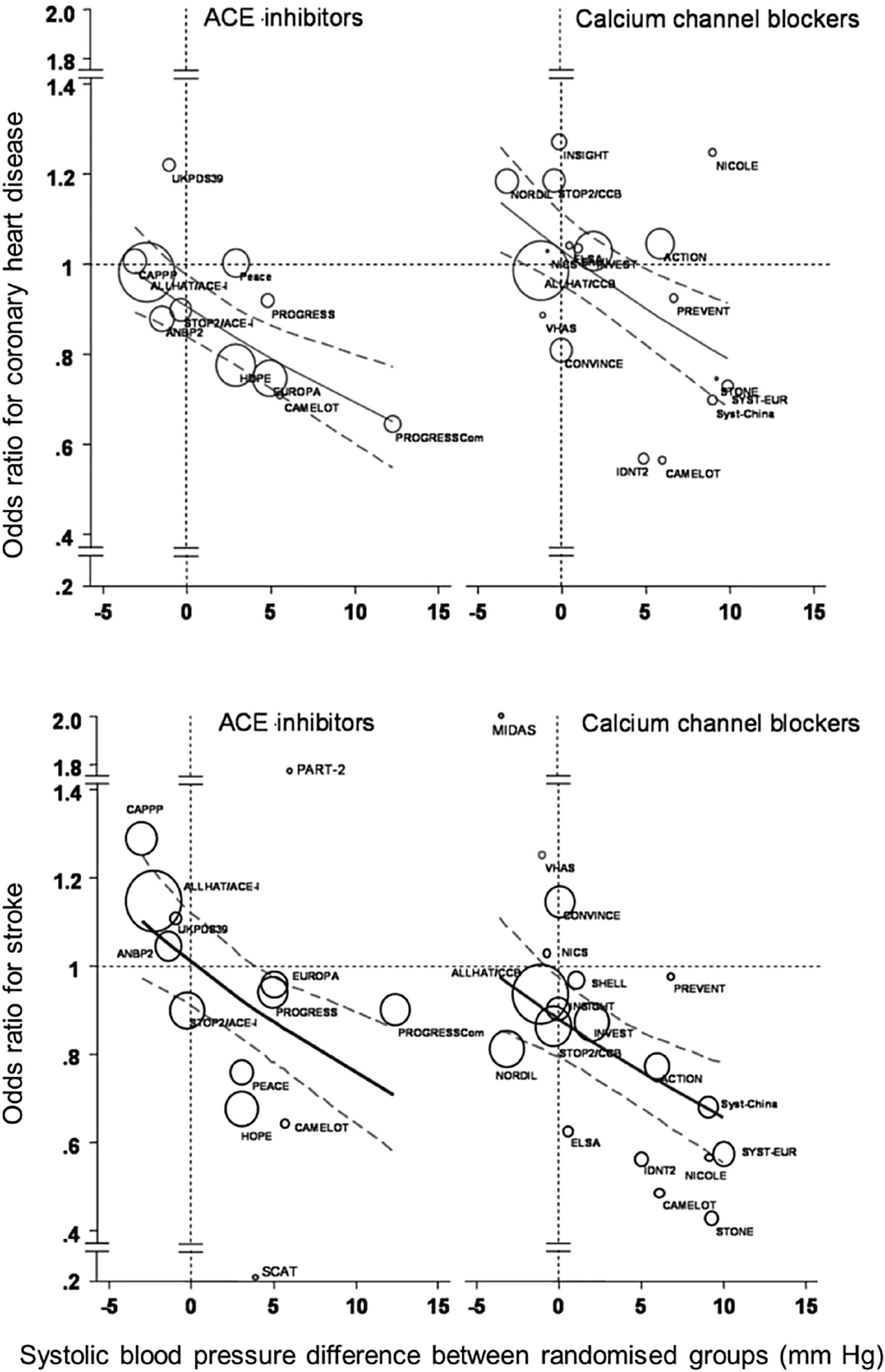
Intrinsic benefits of angiotensin-converting enzyme (ACE) inhibitors in coronary heart disease and calcium channel blockers in stroke. Modified from: Verdecchia et al. Hypertension. 2005;46:386–92.59
Benefits of combinations: synergy at the vascular level
Synergistic effects of drug combinations at a vascular level may explain their synergy at the clinical level.60–64 Most de-stiffening protocols are based on treatment with a RAAS inhibitors plus a diuretic or a CCB. However, because of the inconsistent effect of diuretics in reducing arterial stiffness23 combining a RAAS blocker with a CCB appears a more certain way of reducing PWV. When dissecting further mechanisms of action, those of ACE inhibitors and CCBs are complementary (Fig. 5). Arterial stiffness is regulated by endothelial function.65 ACE inhibitors by preventing the degradation of bradykinin and production of angiotensin II enhance NO bioavailability. RAAS blockers improve endothelial function. In a clinical, mid-term (6 months) study, the effect of antihypertensive drugs (nifedipine GITS, amlodipine, atenolol, nebivolol, telmisartan and perindopril) on conduit endothelial function was tested.66 The ACEi only restored flow-mediated dilation of the brachial artery. Further, it should be noted that enhancement of endothelial function through anti-apoptotic effects is a class effect for ACE inhibitors, but the potency varies, with perindopril having a greater such effect.67 In addition, binding of angiotensin II to AT1 receptors leads to a direct increase in vascular tone and stiffness.68 On the other hand, CCBs prevent smooth muscle cell contraction by blocking receptor- or voltage-operated channels.69
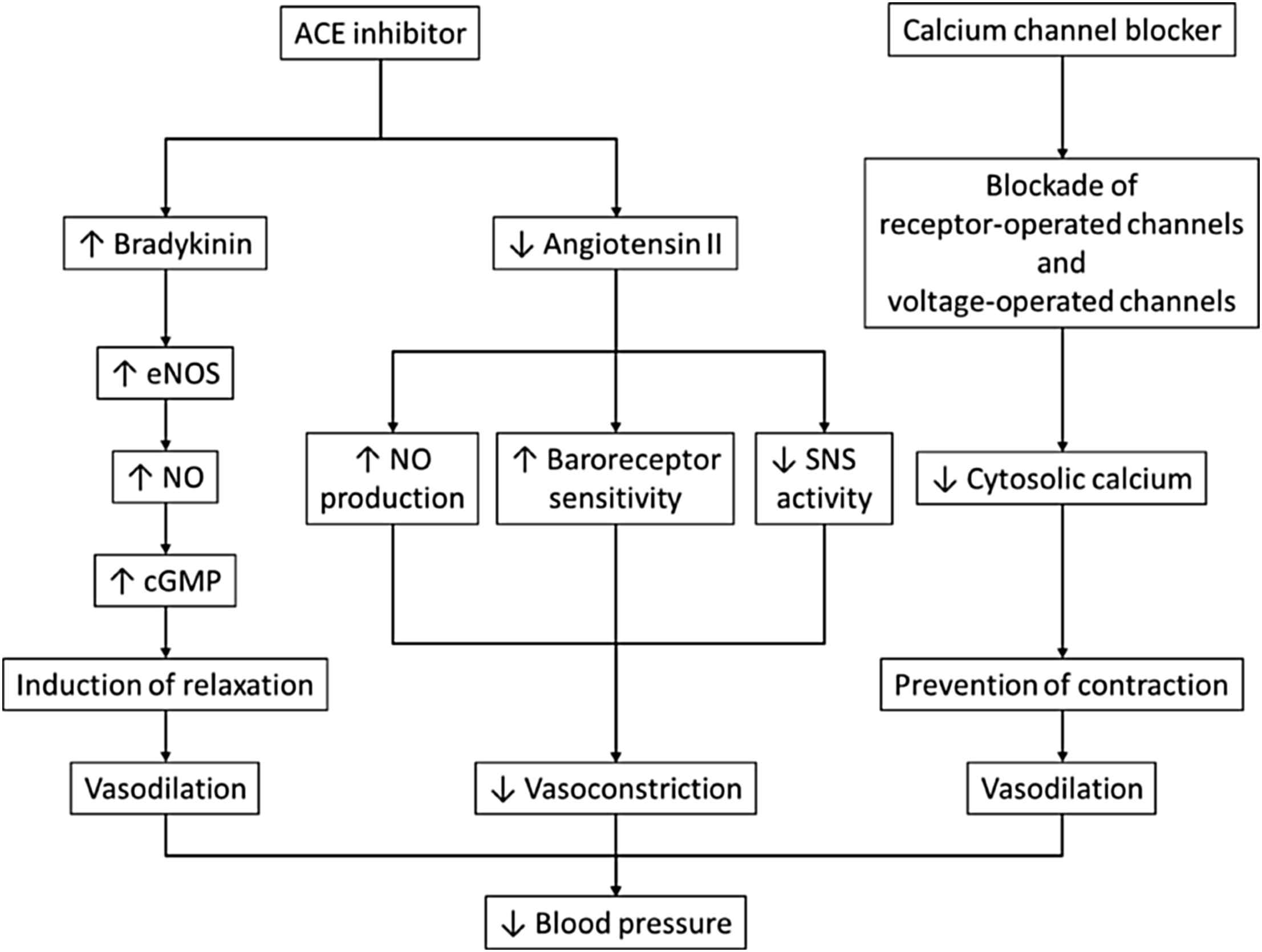
Mechanisms of blood pressure reduction with ACE inhibitors and calcium channel blockers. Modified from: Ferrari. Curr Med Res Opin. 2008;24:3543–57.61 Abbreviations: ACE, angiotensin-converting enzyme; cGMP, cyclic guanosine monophosphate; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; SNS, sympathetic nervous system.
On a long-tem basis, RAAS inhibition can also reduce arterial stiffness by reducing the accumulation of collagen and by increasing the elastin/collagen ratio.70 The expression and activity of matrix metalloproteinases, which damage collagen and elastin, can be reduced with RAAS inhibition, leading to decreased arterial stiffness. Polymorphisms of ACE insertion/deletion and AT1 receptor genes can impact the PWV-reducing efficacy of ACE inhibition.70 Moving further downstream, ACE inhibitors and CCBs have also been shown to reverse structural abnormalities in the small arteries of patients with essential hypertension.71 This impacts beneficially on central haemodynamics by reducing the amplitude of the reflected wave.
Individual ACE inhibitor/CCB combinations: a translational approach
All ACE inhibitor/CCB combinations lower BP effectively, but some may be more advantageous than others for the reduction of CV outcomes.58,60,72 In INVEST (INternational VErapamil-SR/trandolapril Study),72 a regimen based on the CCB verapamil and ACE inhibitor trandolapril had no effect on outcomes over 24 months compared with a regimen based on atenolol and hydrochlorothiazide in hypertensive patients with CAD. However, in the ACCOMPLISH (Avoiding Cardiovascular events through COMbination therapy in Patients LIving with Systolic Hypertension) trial, a combination of benazepril and amlodipine reduced the relative risk of the study’s primary composite CV endpoint by 20% (95% CI, 0.72 to 0.90; p < 0.001) over 36 months in hypertensive patients at high CV risk, compared with benazepril and hydrochlorothiazide.58
A combination of perindopril and amlodipine has also been shown to reduce not only BP62,63 but also CV outcomes in hypertension.60 In ASCOT-BPLA (Anglo-Scandinavian Cardiac Outcomes Trial Blood Pressure–Lowering Arm), a regimen of amlodipine ± perindopril reduced the endpoints of fatal and non-fatal stroke by 23% (p = 0.0003), total CV events and procedures by 16% (p < 0.0001), and all-cause mortality by 11% (p = 0.025), compared with atenolol ± bendroflumethiazide in hypertensive patients with 3 or more CV risk factors after 5.5 years follow-up.60 Importantly, the relative risk of CV mortality was also decreased by treatment with amlodipine ± perindopril (by 24%, p = 0.001), a finding that was not observed with the combination of benazepril/amlodipine in ACCOMPLISH. In alignment, the addition of perindopril to stable CAD patients in EUROPA CCB (EUropean trial on Reduction Of cardiac events with Perindopril in stable coronary Artery disease Calcium Channel Blocker) reduced the primary endpoint (CV mortality, non-fatal myocardial infarction, and resuscitated cardiac arrest) by 35% (p < 0.05) and all-cause mortality by 46% (p < 0.01) versus placebo.64
Individually, perindopril and amlodipine have been shown to positively influence many parameters of pulsatile haemodynamics, including reduction of central BP beyond brachial BP, pulse pressure amplification and augmentation index.23,43,70,73 The intriguing issue with ASCOT was that the favourable effect on outcomes with the combination of amlodipine ± perindopril occurred despite similar peripheral BP reduction with the combination of atenolol ± bendroflumethiazide. The CAFÉ (Conduit Artery Function Evaluation) study,39 a substudy of ASCOT, sought to unravel possible mechanisms by examining the effect on central aortic pressures. Indeed, effect of the two regimen was different: amlodipine ± perindopril significantly reduced central aortic SBP and central aortic pulse pressure more than atenolol ± bendroflumethiazide (by 4.3 mm Hg, 95% CI, 3.3–5.4 mm Hg; p < 0.0001; and by 3.0 mm Hg, 95% CI, 2.1–3.9 mm Hg; p < 0.0001, respectively).
In conclusion, arterial stiffness and central haemodynamics are appealing therapeutic target in patients with elevated cardiovascular risk. Reduction in CV risk with combination therapy, and particularly with using an ACE inhibitor and a CCB, may be mediated through beneficial effects in these biomarkers of early vascular aging.
Conflicts of interest
The author has received honoraria from Servier.
Acknowledgements
The author would like to thank Dr. John Plant for assistance in the writing of this manuscript.
References
Cite this article
TY - JOUR AU - Charalambos Vlachopoulos PY - 2016 DA - 2016/03/05 TI - Combination therapy in hypertension: From effect on arterial stiffness and central haemodynamics to cardiovascular benefits☆ JO - Artery Research SP - 27 EP - 35 VL - 14 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2016.02.005 DO - 10.1016/j.artres.2016.02.005 ID - Vlachopoulos2016 ER -
