The Role of E-health in 24-h Monitoring of Central Haemodynamics and Vascular Function
- DOI
- 10.2991/artres.k.191114.001How to use a DOI?
- Keywords
- e-Health; ambulatory monitoring; pulse wave analysis; pulse wave velocity; central arterial pressure; augmentation index; arterial hypertension; artificial intelligence; machine learning
- Abstract
Recent advances in Pulse Wave Analysis (PWA) technology enable Blood Pressure (BP) measuring devices to combine the non-invasive estimation of different vascular biomarkers in ambulatory conditions. This approach allows obtaining a dynamic assessment of vascular function during the 24-h in the conditions of daily life, including night sleep. In spite of the present limited proof of the prognostic significance of 24-h ambulatory PWA, data is accumulating indicating the ability of these techniques to facilitate the early screening of vascular alterations and to improve individual Cardiovascular (CV) risk stratification. The integration of 24-h PWA with e-health and telehealth may help boost the implementation of this approach in the routine clinical evaluation of patients at risk. Telehealth-based 24-h PWA may help standardize the evaluation of recordings by making available to doctors and researchers validated analytical algorithms through dedicated web services. It may facilitate the setup of a worldwide network between expert centres and peripheral hubs in order to improve the quality of the patient’s assessment and to provide personalized care. It may establish communication between healthcare professionals and patients allowing remote monitoring and direct counselling, ultimately improving patients’ health status. The use of telehealth may also allow creating registries and collecting big-data, useful to validate and improve the quality of the algorithms, including Artificial Intelligence (AI) and Machine Learning (ML) tools for predicting patients’ risk and guide clinical care. Preliminary evidence from one of such registries (the Vascular health ASsessment Of The hypertENSive, VASOTENS Registry) seems to indicate that telehealth-based networks may be effective to collect definitive proof of the clinical utility of 24-h PWA.
- Copyright
- © 2019 Association for Research into Arterial Structure and Physiology. Publishing services by Atlantis Press International B.V.
- Open Access
- This is an open access article distributed under the CC BY-NC 4.0 license (http://creativecommons.org/licenses/by-nc/4.0/).
1. INTRODUCTION
Vascular function and arterial health can be assessed through non-invasive measurement of arterial stiffness, Central Arterial Pressure (CAP) and pulse wave reflections, usually in resting conditions and in the controlled setting of the doctor’s office or of a haemodynamic laboratory [1,2]. Recent advances in technology have now made available non-invasive devices combining peripheral Blood Pressure (BP) measurements and Pulse Waveform Analysis (PWA), making the assessment of arterial functional state more affordable and largely operator-independent, extending the evaluation to the ambulatory conditions and over the 24-h [3]. These tools are more often embedding e-Health solutions which increase their potentials for estimating vascular function in the dynamic conditions of daily life and may provide an opportunity for a more precise individual patient’s Cardiovascular (CV) risk stratification.
In this review, we will present the currently available technologies for 24-h PWA and the evidence for their accuracy and clinical value. We will then discuss the contribution of e-Health, and in particular of telehealth, to the improvement of 24-h monitoring of vascular function and present our experience in the field. Finally, we will briefly introduce the potentials for Artificial Intelligence (AI) and Machine Learning (ML) tools for PWA.
2. AVAILABLE TECHNOLOGIES FOR 24-H PWA
Various non-invasive techniques can allow to record a pulse waveform and then run a PWA in ambulatory conditions [3]. The pulse waveform can be obtained through oscillometry applied to the brachial artery (so-called “cuff-based” approach), or by “cuff-less” techniques which rely upon applanation tonometry, typically at the wrist, or on volume-clamped finger photoplethysmography (often coupled with ECG gating). Then, the recorded waveform is processed by mathematical transformation and the vascular parameters are estimated. As summarized in Table 1, several parameters related to the vascular function can be estimated in ambulatory conditions during non-invasive BP measurements. Some of them can be calculated by combining different estimates in order to obtain a more complex evaluation of the systemic haemodynamics.
| Direct measurement or estimation via specific algorithms | Calculated |
|---|---|
|
|
b, brachial; c, central; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; PP, pulse pressure; HR, heart rate; BP, blood pressure; ECG, electrocardiogram; PWV, pulse wave velocity; QKD, Q wave to Korotkoff delay; PTT, pulse transit time; AIx, augmentation index; SV, stroke volume; CO, cardiac output; SVR, systemic vascular resistance; AASI, ambulatory arterial stiffness index; SI, smoothness index; SD, standard deviation; TOVI, treatment-on-variability index; TPR, trough-to-peak ratio.
Parameters related to the vascular function which can be currently estimated or calculated through pulse wave analysis of non-invasive ambulatory blood pressure recordings
3. ACCURACY OF 24-H PWA
Very recently, 24-h monitoring of central haemodynamics, arterial stiffness and wave reflections in ambulatory conditions has become more and more popular. Several studies evaluated the accuracy of the arterial stiffness parameters provided by the various non-invasive devices or technologies vs. intra-arterial recordings or non-invasive standards. In the majority of studies, measurements of Pulse Wave Velocity (PWV), CAP and Augmentation Index (AIx) were in accordance with the reference standard, though all studies were performed at rest and not in dynamic conditions. Systematic analyses of the accuracy of commercial devices estimating CAP and PWV non-invasively have been published in recent years, providing a more general picture of the current situation. Papaioannou et al. [4], evaluated 22 studies, which validated invasively 11 different commercial devices in 808 study participants (five studies evaluated three different types of devices for use in ambulatory conditions). The overall error in CAP estimation (test vs. intra-arterial reference) was −4.5 mmHg (95% confidence interval: −6.1 and −2.9 mmHg). The accuracy of CAP was slightly better when estimated by oscillometry [−3.2 (−5.5, −0.9) mmHg] than by radial tonometry [−5.4 (−7.6, −3.2) mmHg]. Oscillometric devices with autocalibration function could estimate central Systolic BP (SBP) with a good degree of accuracy [−0.8 (−3.3, 1.7) mmHg]. When other calibration methods were used the most accurate was that relying upon brachial mean arterial pressure and Diastolic BP (DBP) (C2 method) [−3.0 (−5.8, −0.2) mmHg], rather than upon brachial SBP and DBP one (C1 method) [−7.8 (−10.3, −5.3) mmHg].
Very recently, Milan et al. [5], published a systematic review of 26 different invasive and non-invasive studies validating different PWV estimation techniques and assessed the conformity of the results to the Guidelines of the Artery Society and the recommendations of the American Heart Association. The studies included 12 different types of approaches to the estimation of PWV (including Doppler ultrasound and magnetic resonance imaging). Of the evaluated studies, seven were based on three different types of commercial devices to be used for monitoring in ambulatory conditions. The authors found high discrepancies between devices and only six studies (one for an ambulatory recorder) achieved an “excellent” level of accuracy.
Both systematic reviews concluded that due to great heterogeneity among the used methodologies and validation approaches, a consistent uncertainty exists as to whether one device is superior to the other and thus can be recommended as “gold standard” for determining CAP or PWV. Definitely, in the future, it is mandatory to assess the accuracy and clinical validity of each device technology in large scale studies recruiting subjects with different levels of CV risk.
4. CLINICAL IMPACT OF 24-H PWA
The availability of non-invasive operator-independent methodologies for 24-h PWA, particularly of those based on brachial oscillometry, has favoured the diffusion of the estimation of vascular parameters in diverse patient groups and disease states. However, there is currently a significant lack of published evidence validating each technique in specific clinical conditions. Most importantly, although important evidence exists about the long-term predictive power for CV events of vascular indices such as PWV, CAP, and AIx, collected at rest [6–8], this is not the case for the same parameters obtained in ambulatory conditions over the 24-h: the existing studies are limited to high-risk patients with end-stage renal disease. Sarafidis et al. [9] examined the prognostic significance of ambulatory brachial BP, CAP, PWV, and AIx in a population of 170 haemodialysis patients undergoing 48-h Ambulatory Blood Pressure Monitoring (ABPM) and followed-up for 28.1 months. Increasing levels of 48-h ambulatory central Pulse Pressure (PP), PWV, and AIx at baseline were associated with increased risk of CV events and mortality, whereas office SBP and ambulatory brachial and central SBP were not. In a further analysis of the same study, patients with the weaker within-individual association of ambulatory BP with ambulatory PWV displayed a higher risk of death and CV events, suggesting that arterial stiffness may be a promoting factor for adverse outcomes in kidney patients, independently from BP [10].
In the rISk strAtification in end-stage renal disease (ISAR) study [11], including 344 haemodialysis patients, during a follow-up of 36 months, after adjustment for common risk factors, 24-h but not office PWV resulted predictive for all-cause of mortality [hazard ratio and 95% confidence interval: 2.51 (1.31, 4.81); p = 0.004].
5. ADVANTAGES AND LIMITATIONS OF 24-H PWA ASSESSMENT
As summarized above, limited evidence exists about the clinical usefulness of 24-h PWA and, for this reason, clinical guidelines have not yet issued any specific recommendation [12]; nonetheless, the technique has many potential advantages for improving the management of arterial hypertension, as summarized in Table 2. In particular, the fact that these techniques are easy to use and largely operator-independent, and that they extend the evaluation of vascular function to the conditions of daily life, enabling to obtain repeated measurements in different circumstances, are the major advantages.
Advantages
|
Limitations
|
Potential advantages and critical issues related to 24-hour estimates of vascular function
Although the simpleness of the technologies currently available makes the evaluation of vascular function feasible in daily life ambulatory conditions, further research is mandatory prior to the introduction of these techniques in the clinical practice, due to some unsolved critical issues (Table 2). The most important challenge regards the relatively good accuracy and limited proof for clinical effectiveness. Validation studies based either on the gold standard non-invasive technique (Sphygmocor) or intra-arterial measurements have documented strong correlation and acceptable accuracy for most of the tested non-invasive devices. However, the accuracy of the estimation is still largely varying according to the device or technique used.
6. E-HEALTH: GENERALITIES AND POTENTIAL APPLICATIONS TO 24-H PWA
E-Health is a definition including a broad group of activities that rely on communication and information technologies to store, retrieve, share, and exchange health-related information for the management of different clinical conditions, for health education and for administrative purposes. As depicted in Figure 1, e-Health solutions for 24-h PWA are useful at different stages of the signal management process. They basically allow automatic pulse wave identification, appropriate calibration of the recorded waveform, wave separation analysis, modelling by transformation either in the time or frequency domain, derivation of vascular indices by proprietary algorithms or mathematical modelling.
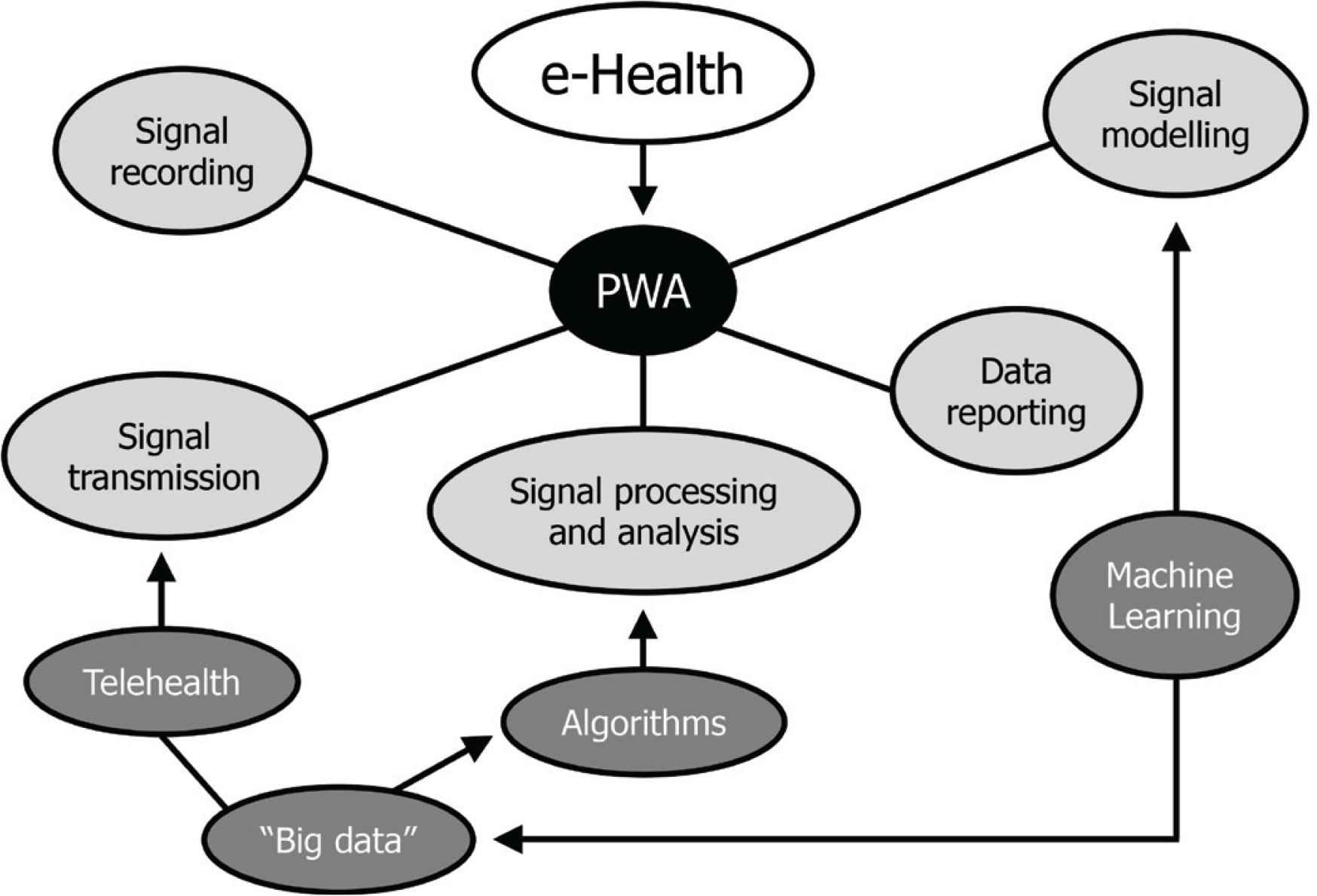
Diagram of typical e-Health application for 24-h pulse wave analysis (PWA).
Telehealth, which is specifically meant to exchange data from one site to the other, is useful at the data transmission level, in order to facilitate communication and to create large data repositories which can be used to feed ML models and tools, as further discussed.
E-Health can help improve 24-h vascular function estimation from the perspective of clinicians and patients and from that of researchers and developers. Major advantages of e-Health applications in the clinical field are: (i) the establishment of an efficient communication between healthcare professionals and patients, through remote monitoring and counselling, with the ultimate goal of improving the quality of delivered care and CV outcomes; (ii) the facilitation of the implementation of vascular function assessment in the routine clinical management of patients at CV risk, and (iii) the setup of a worldwide network between expert centres and peripheral hubs in order to improve the quality of patient’s assessment and to provide personalized care. Potential technical advantages, particularly useful in the research field, include: (i) the provision to researchers and doctors of validated algorithms through dedicated web services (or algorithms libraries available in the cloud); (ii) the standardization and prompt update of algorithms specific to given devices (real-time distribution of software updates), and (iii) the collection of “big data” (for instance through registries) including demographic and clinical data useful to validate and improve the quality of algorithms and to implement ML tools for predicting patients’ risk and guide clinical care.
7. 24-H PWA AND TELEHEALTH: THE VASOTENS REGISTRY
An attempt to provide supporting evidence for the inclusion of telehealth-based solutions for 24-h PWA in routine hypertensive management is currently made by the Vascular health ASssesment Of The hypertENSive (VASOTENS) Registry [13].
In this international, multicentre, observational, non-randomized, prospective study, 24-h ABPMs are currently collected in hypertensive subjects by means of an upper-arm BP monitor enabling combined estimation of some vascular biomarkers through brachial oscillometry. A web-based telemedicine platform allows data collection standardization and centralization, prompt analysis, validation of the results by experts with counselling to remote centres, and setup and maintenance of the Registry (Figure 2). The final goal of this telehealth study is to clinically validate the use of non-invasive 24-h ambulatory PWA for hypertension screening and follow-up, in order to favour the inclusion of this approach in hypertension guidelines.
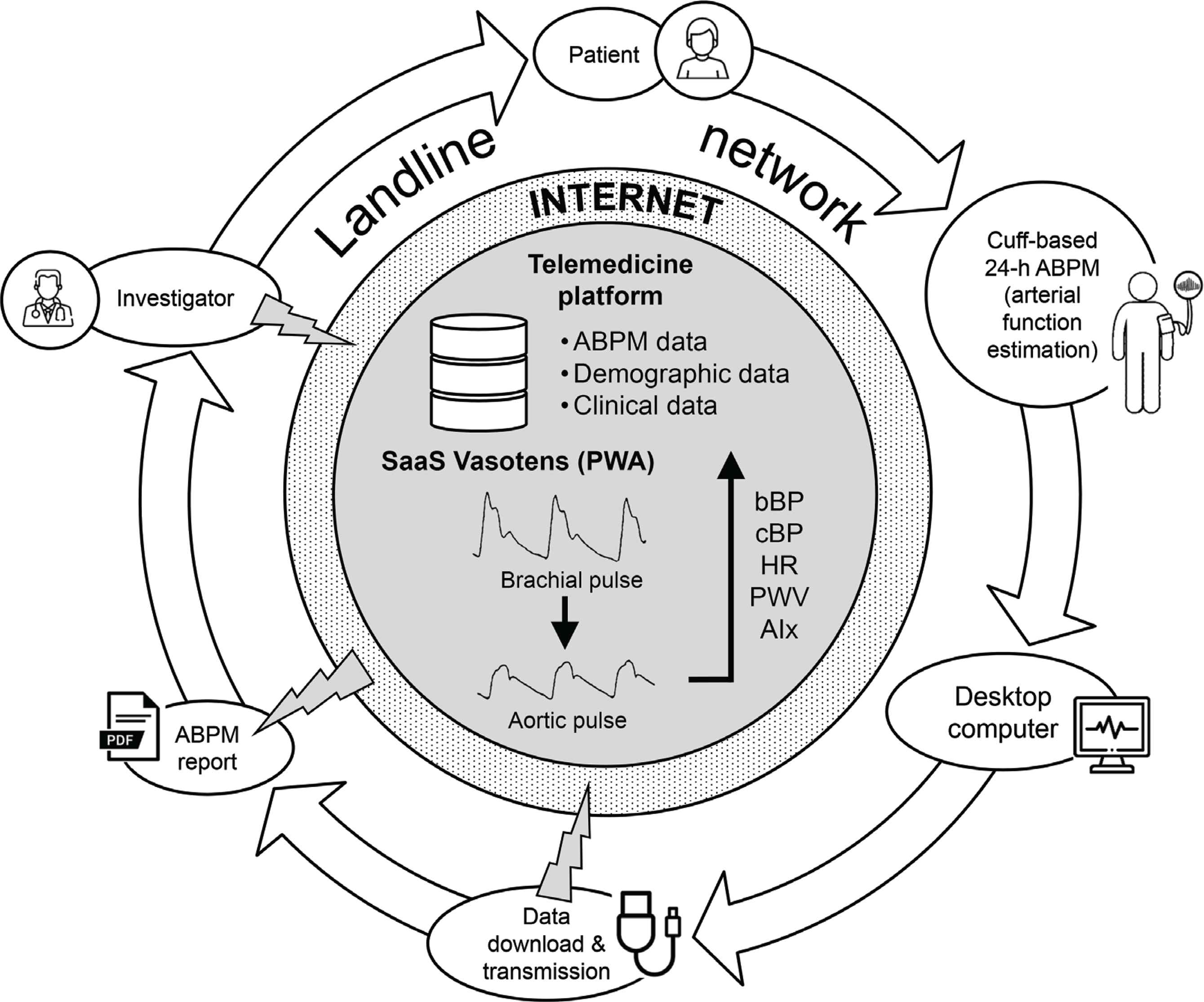
The workflow of the web-based telemedicine system (https://www.tholomeus.net) used in the Vascular health ASssesment Of The hypertENSive (VASOTENS) Registry. ABPM, ambulatory blood pressure monitoring; b, brachial; c, central; BP, blood pressure; HR, heart rate; PWV, pulse wave velocity; AIx, augmentation index; PWA, pulse wave analysis; SaaS, software as a service.
The first publication of the study, reports on the results obtained in 1200 subjects with different levels of CV risk [14]. As shown in Figure 3, after proper adjustment, peripheral and central SBP, PWV and AIx values were significantly increased in older participants and in case of high BP. Brachial and aortic SBPs were significantly lower and PWV and AIx significantly higher in females. PWV was increased in subjects with the metabolic syndrome, in the absence of BP changes, suggesting that this condition is associated with increased arterial stiffness independently of BP. Another publication of the same study, exploring the association of vascular biomarkers with hypertension-mediated organ damage, showed that 24-h brachial and central SBP and PP averages were significantly larger in subjects with than in subjects without left ventricular hypertrophy, whereas no statistically significant difference was observed for these estimates in case of vascular or renal damage [15]. No between-group difference was observed for any type of hypertension-mediated organ damage and PWV, AIx or BP variability. Both analyses confirmed that ambulatory PWA in a daily life setting may help evaluate the vascular health of individuals at risk for CV disease.
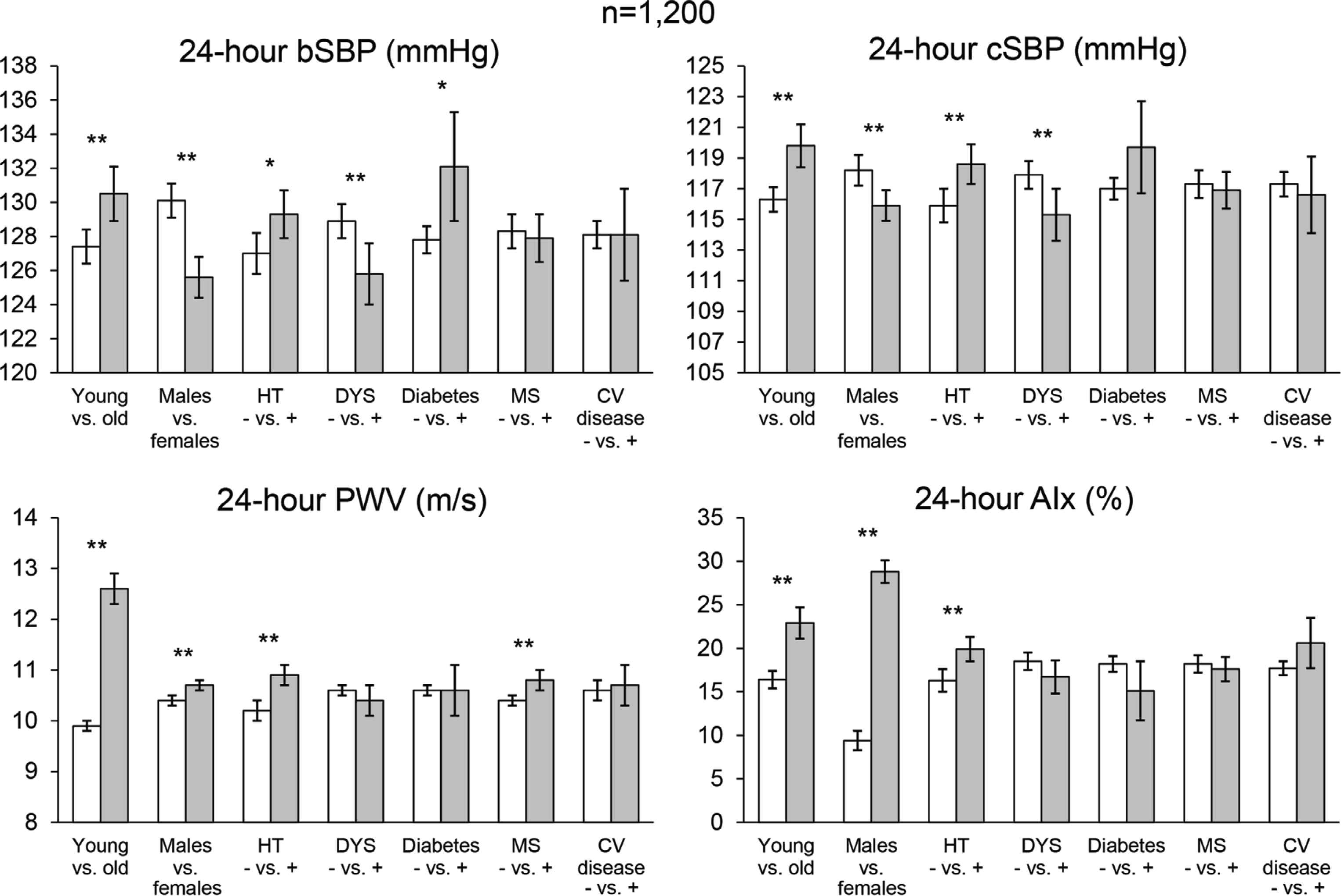
Average values and 95% confidence intervals of 24-h Brachial Systolic Blood Pressure (bSBP), Central SBP (cSBP), Pulse Wave Velocity (PWV) and Augmentation Index (AIx) in 1200 hypertensive patients of the Vascular health ASssesment Of The hypertENSive (VASOTENS) Registry. Data are shown in specific high-risk subgroups. bSBP, cSBP, and PWV estimates are corrected by age, sex, cardiovascular risk factors (including hypertension, diabetes, dyslipidaemia, cardiovascular disease) and obesity. AIx is also adjusted by SBP. p-Values of the comparisons within each subgroup are reported (**p < 0.01; *p < 0.05). HT, hypertension; DYS, dyslipidaemia; MS, metabolic syndrome; CV, cardiovascular (redrawn from Omboni et al. [14] by permission).
8. THE POTENTIAL FOR ARTIFICIAL INTELLIGENCE AND ML FOR PWA
A major challenge that e-Health is actually facing in the era of “Internet-of-Medical-Things” is the correct handling, interpretation, and use of data about the individual patient and his/her condition that the new approach based on digital networks, centred on the person, generates. In this regard, AI conveniently and appropriately applied to the data managed at different levels may provide a solution. AI roughly refers to algorithms that are able to learn from data without being programmed on purpose where to look [16]. ML is a type of AI based on pattern recognition, consisting of algorithms relating all or some predictors to an outcome [17]. In order to estimate the model, the ML algorithms randomly or deterministically seek for the best fit. Basically, ML is a “data-driven” analytical strategy, which must be considered as a cognitive extension of physician’s intervention, augmenting his/her potentials for making patient-specific decisions rather than replacing his/her intervention.
Table 3 summarizes the currently available evidence from the literature of application of ML to 24-h PWA. The most relevant utility of ML in this field is the possibility to estimate pulse wave parameters with no need for calibration over time or according to the subject, and thus independently of the person whose values are being measured. Two interesting examples of this application are shown in Figure 4. In the first study, the use of an ML model based on Artificial Neural Networks (ANN) allowed to estimate invasive aortic SBP from radial SBP, DBP, and Heart Rate (HR) alone (without recording a peripheral waveform) in a way comparable with a validated Generalized Transfer Function (GTF) [18]. The estimated aortic SBP for all ANN methods was on average within 2 mmHg of measured aortic SBP, whereas variability of the difference tended to be high, ranging approximately between ±4 and ±6 mmHg, depending on the ANN model used. The technique may thus provide plausible aortic SBP estimation without waveform analysis.
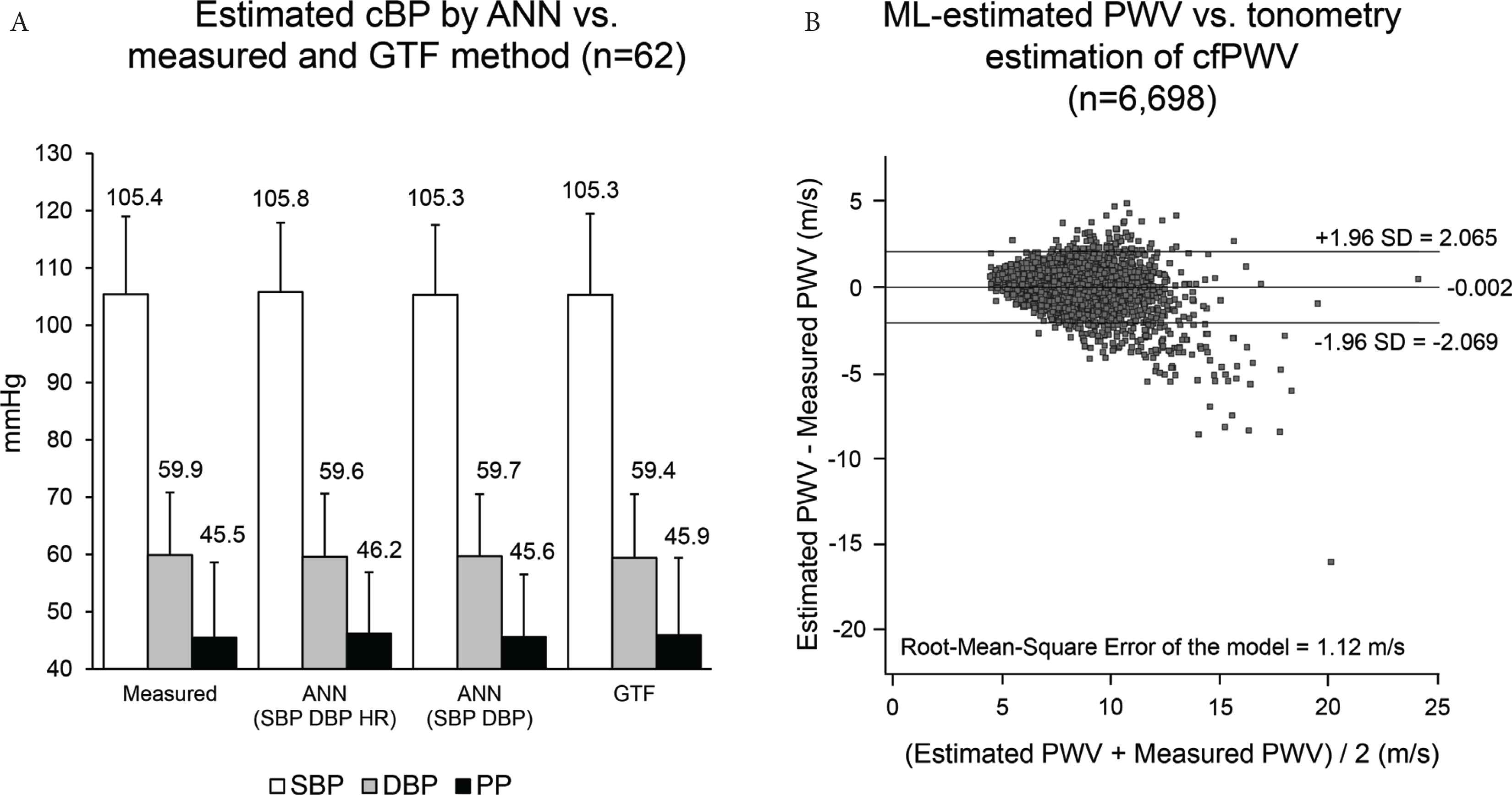
(A) Average ± standard deviation (SD) of invasively measured Central Blood Pressure (cBP) and of that estimated by an Artificial Neural Network (ANN) and Generalized Transfer Function (GTF) [redrawn from Xiao et al. [18] by permission]. (B) Bland–Altman plot of the difference between estimated and measured values of carotid-femoral pulse wave velocity (PWV, y-axis) vs. the average of the two PWVs (x-axis). The values for the average difference ± 1.96 SDs are also shown. SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; HR, heart rate; ML, machine learning [redrawn from Tavallali et al. [19] by permission].
| Feature | Evidence (number of studies) |
|---|---|
| BP (SV or CO) estimation from invasive or non-invasive continuous pulse waveforms, from ECG or from oscillograms, and from PTT (“calibration-free”) | ••••• (25) |
| Disease or risk factors identification/stratification from PWA, including estimation of biological/vascular age and of BP normality | •• (10) |
| Prediction of risk from multiple derived parameters and nomograms based on decision tree analysis (“big data” analysis) | • (3) |
| cfPWV estimation from carotid pulse waveform or wave reflections in the aorta estimated at the radial artery | • (2) |
| Detection of (motion) artefacts and reduction of uncertainty of pulse wave detection | • (3) |
| Detection of acute events from PWA (e.g. hypotension) | • (3) |
BP, blood pressure; SV, stroke volume; CO, cardiac output; ECG, electrocardiogram; PTT, pulse transit time; cfPWV, carotid-femoral pulse wave velocity.
Main published research on machine learning related to pulse wave analysis (PWA)
In another article, Tavallali et al. [19] evaluated whether an ML approach could estimate the carotid-femoral PWV from one uncalibrated carotid waveform measured non-invasively by tonometry and using few routine clinical variables (age, sex, blood lipids, smoking, diabetes, PP). The dataset consisted of 6698 recordings of a test population with an age ranging between 20 and 69 years. The method performs intrinsic frequency analysis of the carotid waveform and then it models the PWV by ANNs through bootstrap averaging. The model estimated PWV with an error of 1.12 m/s compared with the reference method, with more errors for larger PWV values.
The data-driven approach of ML may also help optimize the algorithms of PWA by comparing predictions with data simultaneously obtained through reference standards (typically intra-arterial measurements) and improve the quality assessment of the pulsatile signals. Application of deep-learning analysis (a specific type of ML) to “big data” collected through registries may help improve the patient risk stratification and allow accurate long-term risk prediction.
9. PERSPECTIVES AND CONCLUSION
Twenty-four-hour PWA seems a promising tool for evaluating vascular function and damage in daily-life conditions and promoting early screening in subjects at risk. Unfortunately, there is limited proof of its accuracy, diagnostic quality, and clinical value, which all seem to be strongly “device-dependent”. For this reason, we urge to collect a large amount of data, better if outcome-based, to provide reference clinical value for each device/algorithm. e-Health may help to collect definite evidence of clinical effectiveness of 24-h PWA and boost and disseminate solutions based on 24-h PWA by interconnecting different users. Ideally, e-Health should help provide users with new accurate “device-independent” tools for data modelling and analysis, thanks to the use of AI and ML.
CONFLICTS OF INTEREST
The author is a scientific consultant of Biotechmed Ltd. provider of telemedicine services.
FUNDING
No funds or grants were received for the preparation of this manuscript.
Footnotes
REFERENCES
Cite this article
TY - JOUR AU - Stefano Omboni PY - 2019 DA - 2019/11/21 TI - The Role of E-health in 24-h Monitoring of Central Haemodynamics and Vascular Function JO - Artery Research SP - 11 EP - 17 VL - 25 IS - 1-2 SN - 1876-4401 UR - https://doi.org/10.2991/artres.k.191114.001 DO - 10.2991/artres.k.191114.001 ID - Omboni2019 ER -
